January 17, 2022 feature
What does myelin actually do?
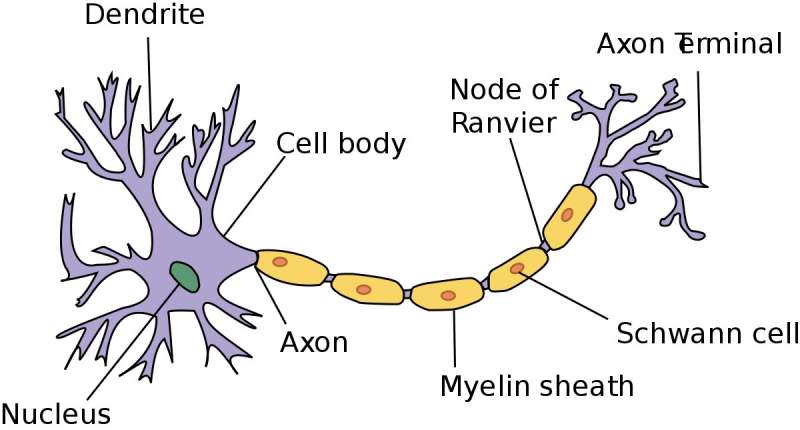
Students of physiology are invariably taught that the primary function of myelin is to insulate nerves. In particular, to make action potentials more efficient by increasing the thickness of the membrane and thereby decreasing its electric capacitance. But this crude idea—this analogy, really—can't be correct. Despite the protestations of dogmatic neuroscientists, neurons aren't electric devices, at least not in the sense of electrons flowing in wires.
Sure, there appear to be plenty of electron currents flowing through and between proteins in the inner membranes of mitochondria deep inside of neurons (some say up to 50 amps for the whole body), but these currents have nothing to do with action potential propagation. Spikes are multifaceted biophysical disturbances in the axons. They clearly do have an ionic component in the form of assorted flows of sodium, potassium, chloride, calcium, and potentially even protons, through channels and pumps; however, electrons are not the bearer of any current or conductance here.
So then what does myelin actually do for an axon? One popular answer has been that it provides some kind of energetic or trophic support, perhaps much like a mitochondrion of sorts that could produce ATP through oxidative phosphorylation via ectopically expressed respiratory complexes. There is a fascinating literature describing the presence of presumably functioning respiratory complex V, the F1FO-ATP synthase, outside of mitochondria in places like rod outer segments in the retina and in myelinating cells. This may not be all that surprising in light of the presence of several kinds of ATP-ases found in different cell compartments.
It is seemingly quite impossible for cells to assemble full-blown respiratory complexes outside of mitochondria due to the need for in-house construction, and subsequent membrane insertion of the extremely hydrophobic mitochondrially expressed proteins, and the extensive intra-mitochondrial processing and maturation of the nuclear-derived protein subunits (see, for example, this critique of allotopic expression of mitochondrial proteins). Nonetheless, there are other ways in which these proteins might find their way to the plasma membrane once they have been assembled. Creative destruction of parts of mitochondria, with the formation of all manner of single- and double-hulled budding vesicles, could conceivably ferry complexes to the border and beyond.
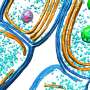
In the current issue of the Royal Society's Open Biology, Alessandro Morelli et al. present intriguing evidence that myelin sheaths with concentric multilamellar structures possess similar bioenergetics to cyanobacterial thylakoids. In addition to a host of eclectic molecular refinements held in common, both structures also share the apparent function of feeding nutrients, potentially including ATP-synthase-derived ATP, into the central heart of a complex multilamellar structure. No one is claiming myelin itself was derived from thylakoids membranes, as that would seem taxonometrically impossible, only that these observations may comprise an enlightening example of convergent evolution to fulfill some fundamentally similar task.
Beyond gross structure and ATP generation, there are other clues to this common underlying function. For example, tightly packed concentric lipids reliably appear as nature's optimal construction for dissolving and sequestering the largest amount of gas in a particular volume. Lipids, particularly neutral lipids, can hold about five times as much gas as water. In this case, the cyanobacteria would be most interested in dissolving CO2 for carb construction, and nitogen for fixation, while myelin would no doubt be seeking O2. Brain tissues do not have the luxury (as do other high-respiring tissues like muscle) of having a high-affinity myoglobin to snatch away O2 from circulating hemoglobin.
It may be interesting to compare coronal sections of cetacean brain with that of the human in light of the vastly thinner and more convoluted cetacean cortex that has evolved under the selective pressure of oxygen deprived conditions. Cetacean cortex may likely contain a large preponderance of large deep layer projection neurons relative to their thin upper layers in order to maximize the number of axons available for producing white matter. A cursory look at dolphin brains on Google images does not support this one way or the other, however a more exhaustive accounting could be illuminating. Similarly, the spermaceti of the sperm whale, which presumabkly beamshapes and focuses ingoing or outgoing echolocation signals, may moonlight in an oxygen nourishing capacity. Their wax par excellence, i.e. cetyl palmitate is a C16 fatty acid that esterifies an alcohol and may be deal for absorbing oxygen. Curiously, concentric multilamellar structures are also produced in the lung surfactant, the crucial element for good O2 absorption by the pulmonary alveoli. The outermost layer of pulmonary epithelial cells also have similar lamellar body formations.
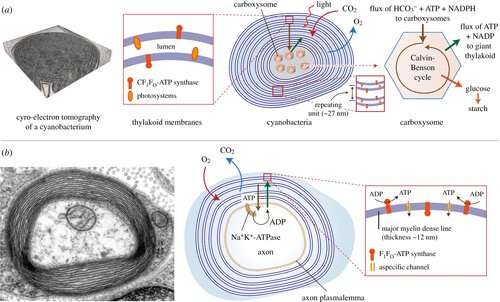
In order to overcome the negatively charged repulsive forces of phospholipids in multilamellar structures, nature seems to have unanimously gone with galactolipids. These lipids, which have galactose as their sugar group, are a favorite among plant membrane lipids, where they substitute for phospholipids presumably to conserve phosphate for other essential processes. Galactolipids make up about 70 percent of the lipids in the cyanobacteria thylakoids, up to 80 percent in plant thylakoids, and about 30 percent in myelin. Myelin galactolipids have also been shown to be essential for proper formation of the nodes of Ranvier.
Another commonality with thylakoids is a close homology in the protein sequences of non-selective ion channels in thylakoids with the ubiquitous, voltage-dependent anion channels (VDAC) abundantly expressed in mitochondria and other membranous structures. In myelin, the VDAC can potentially tetramerize into complexes which would form a central pore approximately 1.3 nm in diameter, roughly the same size as the thylakoid ion-channel pore. In the green unicellular alga Chlamydomonas reinhardtii, these channels connect the thylakoid stacks to an essential structure called the pyrenoid. Bicarbonate, NADPH and ATP are all conducted through pores to the central pyrenoid to feed the Calvin–Benson cycle.
A very high concentration of the enzyme RuBisCo is required for CO2 incorporation into organic compounds. While being the most abundant protein in our entire biosphere, RuBisCo displays only modest catalytic efficiency. This is the likely reason that the dense nuclei of RuBusCo complexes which form carboxysome are found deep within the centers of concentric lamellar structures. To transport CO2 to the business end of the thylakoids, carbonic anhydrase is required to form a bicarbonate intermediate, much as it is in us. Carbonic anhydrase is essential in mitochondria to convert Krebs cycle derived CO2 into bicarbonate. Another place where carbonic anhydrase is found is the myelin sheath, suggesting a metabolic role in handling this gaseous species.
Mitochondria contain expansive membrane compartments known as cristae. Normally these elements form parallel stacks sporadically connected to the outer membrane by tubular cristae junctions. Under certain circumstances, these cristae can completely reconfigure themselves into concentric, onion-like membranes. Many manipulations, both natural and artificial, cause mitochondria to adopt concentric cristae, including alterations in ATP-synthase expression. Some time ago, a few researchers suggested that myelin acts like a mitochondrion. A theoretical objection to this concept later claimed that if an errant ATP-ase should find its may into myelin, at best, it could only operate in reverse to break down ATP.
These author's calculations for the proton motive force across the myelin membrane were based on known values for pH and the membrane potential of the oligodendrocyte, and a few assumptions about the underlying configuration and polarity of the ATPsynthase in the membrane. Theoretical proofs of metabolic this-or-thats, like the amount of energy required by neurons, are notoriously difficult—nature invariably surprises with how much it can do with so little. For example, the same researchers throwing shade on the myelin hypothesis have also made noble attempts to calculate the energy requirements for spiking axons. While many stealth energy sources are also available to the axon, like vesicle-attached ATP generators in the form of GADPH, axons do a lot more than spike. In fact, spiking may be relatively easy compared to more physical processes like transport and growth.
Peter Mitchell, of Nobel fame, introduced the concept of H+-motive force (typically around 250 mV), which would be constituted by the transmembrane electrical potential difference (Δψ) plus the pH difference between the two aqueous phases (ΔpH). However, in talking to Alessandro, an extant pioneer tangibly connected to that bountiful era, and still quite active today, it quickly becomes apparent that all may not be well in chemiosmotic land. It has been observed that the biological membrane surface is separated from the bulk aqueous phase by ordered water molecules representing an electrostatic barrier, which for H+ ranges around 120 meV. Any calculation of the value of pmf across different surfaces is therefore far from straightforward when the actual biology is considered.

The nature of free protons in and around membranes is similarly difficult to intuit. A particularly high proton concentration probably wouldn't be very healthy for membranes. With better techniques, physical variables in structures like mitochondria can now be measured with greater spatial and temporal accuracy, often with astounding results. Temperatures deep inside the matrix literally blaze, calcium sparks fire, and mitochondrial membrane potentials slew about within an order of magnitude of the speed of the spikes of the parent axon itself.
More information: Alessandro Maria Morelli et al, Myelin sheath and cyanobacterial thylakoids as concentric multilamellar structures with similar bioenergetic properties, Open Biology (2021). DOI: 10.1098/rsob.210177
© 2022 Science X Network