Study shows life-saving benefit of baricitinib for ventilated COVID patients
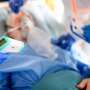
Critically ill COVID-19 patients on a mechanical ventilator or extracorporeal membrane oxygenation (ECMO) lived more often when randomized to receive baricitinib. Doctors call this drug "bari," and receiving the pill once a day for up to 14 days yielded one of the largest a survival advantages seen yet in the COVID pandemic, according to a study published in The Lancet Respiratory Medicine.
It is the first randomized study of the drug in ventilated ICU patients, half of whom received the study drug while the other received placebo. It is also the very drug predicted most likely to benefit COVID-19 patients in a recent AI study to determine which drugs could likely be repurposed to treat COVID-19.
"This study found that among COVID patients who were already critically ill on a mechanical ventilator, adding baricitinib to usual care (steroids) saved lives," said first author E. Wes Ely, MD, professor of Medicine and Critical Care at Vanderbilt University Medical Center and associate director of aging research at the Nashville VA GRECC.
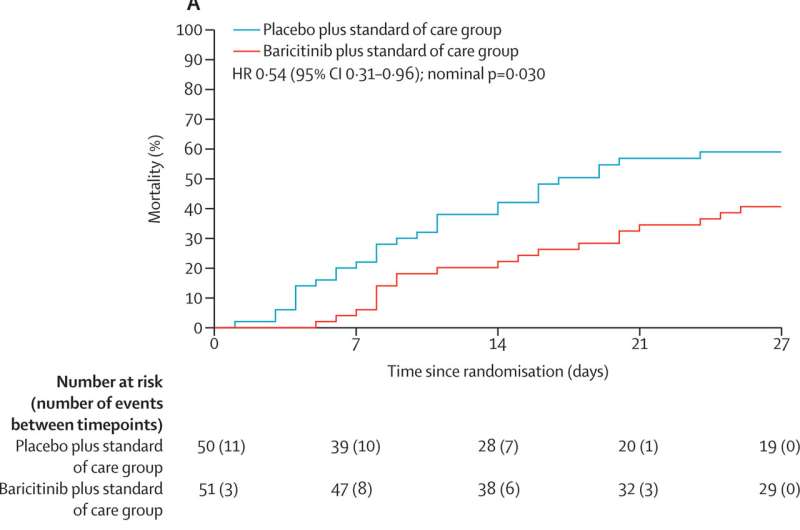
"At 60 days, 62 percent of patients given placebo had died versus only 45 percent of those receiving bari. This means that for every 6 people treated with bari as opposed to the placebo, one additional life was saved," he said.
Baricitinib is an oral, selective Janus kinase (JAK) 1,2 inhibitor used in the treatment of rheumatoid arthritis that has previously shown efficacy in studies of hospitalized adults with COVID-19, including the COV-BARRIER multinational trial with study patients enrolled from 101 sites in 12 countries.
In designing this investigation, Ely partnered with Vince Marconi, MD, professor of Infectious Diseases at Emory University. Marconi and colleagues had been interested in using JAK inhibitors for more than a decade to suppress inflammation in HIV. When the computer determined baricitinib was the prime suspect to save lives in COVID-19, it set the physician-scientists in motion with other collaborators at Eli Lilly and around the world.
"These data offer us a new tool in our armamentarium to help people live even if they are sick enough to require a breathing machine," Ely said.
The study took place from Dec. 23, 2020–April 10, 2021, enrolling 101 participants with 51 receiving baricitinib plus standard of care and 50 receiving placebo plus standard of care.
Treatment with baricitinib significantly reduced all-cause mortality when compared with placebo as 29 of the 50 participants (58 percent) died in the placebo group versus 20 of the 51 participants (39 percent) in the baricitinib group.
"This was a fairly small study of 100 patients done alongside our large phase III study of 1,525 patients in which we found similar life-saving benefit for less sick hospitalized patients," Ely said.
"Next, we need more clinical trials designed explicitly to determine how to improve survival for this subset of the world's sickest COVID patients."
The World Health Organization (WHO) recently updated its guidelines to 'strongly recommend' baricitinib in combination with steroids to treat severe or critical COVID-19 patients in a recent BMJ article.
More information: Zosia Kmietowicz, Covid-19: WHO recommends baricitinib and sotrovimab to treat patients, BMJ (2022). DOI: 10.1136/bmj.o97
Peter Richardson et al, Baricitinib as potential treatment for 2019-nCoV acute respiratory disease, The Lancet (2020). DOI: 10.1016/S0140-6736(20)30304-4
E Wesley Ely et al, Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial, The Lancet Respiratory Medicine (2022). DOI: 10.1016/S2213-2600(22)00006-6