Neuronal mechanism that controls anxiety-like behavior and energy expenditure
Stress responses including physiological and behavioral changes facilitate threat avoidance. Long-term stress affects normal brain function and induces both anxiety and glucose or lipid metabolism disorders, leading to the development of a series of physical and mental illnesses.
The ventromedial hypothalamus (VMH) is an evolutionarily conserved deep subcortical nucleus, and its dorsomedial (dm) region is specifically involved in maintaining energy homeostasis and in the response to stress. A recent study has suggested that the dmVMH might be a crucial central node that connects the regulation of emotion and metabolic processes.
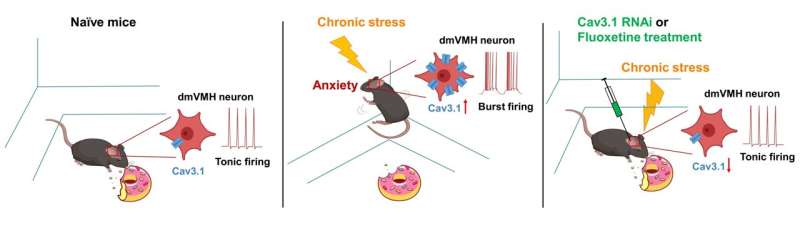
Researchers led by Prof. Yang Fan from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences recently revealed the important role of Cav3.1-driven burst firing of dmVMH neurons in controlling anxiety-like behavior and energy expenditure after chronic stress. Cav3.1 is one isoform of T-type calcium channels that are regarded as a key mechanism underlying neuronal burst firing.
Their study was published in Molecular Psychiatry on Mar. 22.
In this study, the researchers found enhanced burst firing in the dmVMH after chronic stress, and the strength of the burst firing was correlated with anxiety-like behavior. Optogenetically evoked burst firing in dmVMH neurons could induce anxiety-like behavior, and the reduction of food intake and energy expenditure.
"We expressed optogenetic genes in dmVMH neurons and mimicked the burst firing enhancement in vivo by applying wireless optogenetics. This manipulation was sufficient to simulate the anxiety-like behavior and metabolic changes occurring after chronic stress," said Prof. Yang.
The team investigated the molecular mechanism underlying dmVMH burst firing enhancement after chronic stress and found elevated expression levels of Cav3.1 in the dmVMH of stressed mice.
"Injection of the calcium channel inhibitor mibefradil into the dmVMH could relieve the anxiety-like behavior, as well as the reduction in food intake and energy expenditure induced by chronic stress," said Prof. YANG.
The researchers also developed a dmVMH-specific RNA interference method to decrease Cav3.1 expression in stressed mice and suppress burst firing in the dmVMH. These Cav3.1-knockdown mice displayed a significant difference in anxiety-like behavior, food intake and energy expenditure compared with the stress group.
In addition, long-term application of fluoxetine (a serotonin selective reuptake inhibitor) also inhibited the expression of Cav3.1 and mitigated the behavioral and metabolic changes in chronically stressed mice.
"Fluoxetine has been widely used in the clinical treatment of anxiety disorders," said Prof. Yang. "We found fluoxetine could relieve phenotypes of chronically stressed mice via inhibiting VMH burst firing, which further indicated the important role of dmVMH burst firing in the regulation of anxiety and energy expenditure."
This study sheds light on the molecular and cellular mechanisms underlying stress-induced anxiety-like behavior and energy expenditure disorders. It also provides potential intervention targets for treating chronic stress-induced emotional and metabolic disorders.
More information: Jie Shao et al, Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure, Molecular Psychiatry (2022). DOI: 10.1038/s41380-022-01513-x