CircRNA rtcisE2F regulates self-renewal of liver tumor-initiating cell by crosstalking with RNA m6A modification
Liver cancer is one of the most serious cancers in China. Tumor-initiating cells (TICs), a small subset of cells in the tumor bulk with self-renewal and differentiation capacities, play key roles in tumor formation, metastasis, drug resistance and recurrence. circRNAs, formed by covalent conjugation of 5' and 3' ends through backsplicing, play important regulatory roles in many physiological and pathological processes. However, the role of circRNAs in liver cancer and TICs needs further study.
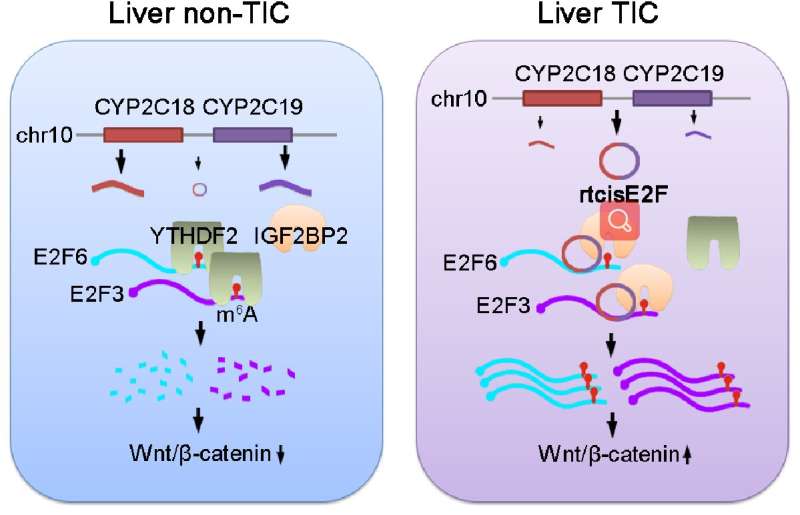
Recently, the groups of Pingping Zhu (School of Life Sciences, Zhengzhou University) and Benyu Liu (The Academy of Medical Science, Zhengzhou University) have identified a circrNA, termed rtcisE2F, is highly expressed in liver TICs. rtcisE2F plays an essential role in the self-renewal of liver TICs. rtcisE2F targets E2F6 and E2F3 mRNAs, attenuates mRNA turnover, and increases E2F6/E2F3 expression. Mechanistically, rtcisE2F functions as a scaffold of m6A reader IGF2BP2 and m6A modified E2F6/E2F3 mRNA. rtcisE2F promotes the association of E2F6/E2F3 mRNAs with IGF2BP2, and inhibits their association with another m6A reader, YTHDF2. IGF2BP2 inhibits E2F6/E2F3 mRNA decay, whereas YTHDF2 promotes E2F6/E2F3 mRNA decay. By switching m6A readers, rtcisE2F enhances E2F6/E2F3 mRNA stability. E2F6 and E2F3 are both required for liver TIC self-renewal and Wnt/β-catenin activation, and inhibition of these pathways is a potential strategy for preventing liver tumorigenesis and metastasis.
Although there have been some studies on the roles of m6A modification and circRNAs in tumorigenesis and tumor metastasis, there have been few reports on the interaction between m6A modification and circRNA, and systematic studies on the interaction between m6A and circRNA in liver tumor and TICs are still lacking. The new findings of Pingping Zhu and Benyu Liu reveal that circRNA play a key role in the binding of m6A modified mRNA to different m6A readers. The rtcisE2F -IGF2BP2 /YTHDF2-E2F6/E2F3-Wnt/β-catenin pathway has been found for the first time to drive the self-renewal of liver TICs, and the tumorgenesis and metastasis of HCC as well. Moreover, these discoveries may provide an additional strategy to eliminate liver TICs.
The work is published in Science China Life Sciences.
More information: Zhenzhen Chen et al, rtcisE2F promotes the self-renewal and metastasis of liver tumor-initiating cells via N6-methyladenosine-dependent E2F3/E2F6 mRNA stability, Science China Life Sciences (2022). DOI: 10.1007/s11427-021-2038-5