Studying intranasal human milk as stem cell therapy in preterm infants with intraventricular hemorrhage
A new study demonstrates that intranasal human milk is a safe and feasible intervention for intraventricular hemorrhage, a serious cause of morbidity in preterm infants. Findings from the study will be presented during the Pediatric Academic Societies (PAS) 2022 Meeting, taking place April 21-25 in Denver. This is the first prospective trial on safety and feasibility of intranasal human milk administration in neonates with intraventricular hemorrhage.
Intraventricular hemorrhage is a common cause of brain injury with potential resultant neurodevelopmental sequalae for preterm infants. Stem cell therapies are being developed as a novel brain injury treatment. Fresh human milk contains pluripotent stem cells that produce neuronal cells in vitro. Animal models of neonatal brain injury have found milk stem cells in brain tissue of suckling mice with neuroprotective effects. Nasal vascularity and the permeable neonatal blood brain barrier potentially allow stem cell delivery to brain tissue via fresh intranasal human milk administration.
"Our study, 'Intranasal Human Milk as Stem Cell Therapy in Preterm Infants with Intraventricular Hemorrhage: Safety, Feasibility and Short-Term Outcomes,' attempts to harness the power of fresh human milk, which has stem cells and growth factors, as an intervention for very preterm babies with brain bleeds, a condition currently with no effective therapy," said Alessia Gallipoli, MD, neonatal-perinatal medicine fellow at the University of Toronto. "Our prospective trial in Toronto, the first in the world, showed that these preterm infants were able to tolerate nasal milk therapy through 28 days of life without major safety events. We hope to use this preliminary data to plan a larger trial to gain more information on the effect of intranasal breastmilk on short- and long-term outcomes for this patient population."
-
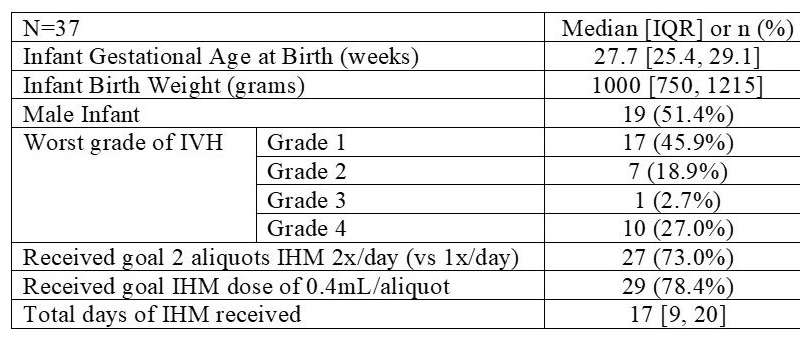
Patient Characteristics and IHM Dosing Information IVH: Intraventricular hemorrhage; IHM: intranasal human milk. Credit: University of Toronto Temerty Faculty of Medicine -
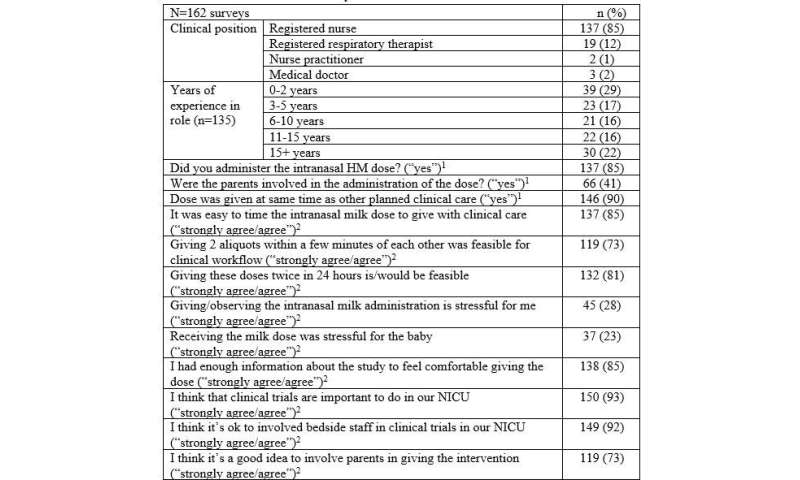
Clinical Provider Survey Results HM: human milk; NICU: neonatal intensive care unit; (1) survey answer options: yes/no; (2) survey answer options: strongly agree/agree/neutral/disagree/strongly disagree. Credit: University of Toronto Temerty Faculty of Medicine
Short-term outcomes are currently being compared to a historical control. Next steps will explore long-term neurodevelopmental outcomes at 18 months to plan for larger multicenter trials.
More information: Conference: www.pas-meeting.org/




















