Post-COVID lung disease shares origins with other scarring lung disorders
While most people recover from COVID-19 within a week or two, up to one-third of survivors experience persistent or new symptoms weeks and months after initial infection.
One form of "long COVID" is interstitial lung disease (ILD), a group of chronic pulmonary disorders characterized by inflammation and scarring (fibrosis) that make it hard for the lungs to get enough oxygen. Little is currently known about ILD, from diagnosis to prognosis to management. In its most severe form, the disease is fatal without a lung transplant.
In a new study, published in the July 20, 2022 online issue of eBioMedicine, researchers at University of California San Diego provide the first insights into the fundamental cellular pathologies that drive ILD.
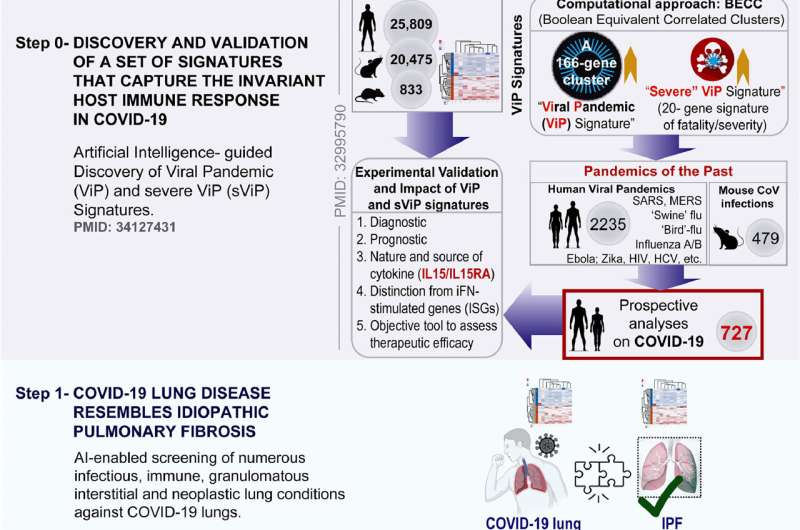
"Using an artificial intelligence (AI) approach, we found that lung fibrosis caused by COVID-19 resembles idiopathic pulmonary fibrosis (IPF), the most common and the deadliest form of ILD," said co-senior study author Pradipta Ghosh, MD, professor in the departments of Medicine and Cellular and Molecular Medicine at UC San Diego School of Medicine. "At a fundamental level, both conditions display similar gene expression patterns in the lungs and blood, and dysfunctional processes within alveolar type II (AT2) cells."
AT2 cells play several critical roles in pulmonary function, including the production of lung surfactant that keeps lung cells from collapsing after exhalation and regeneration of lung cells after injury.
"The findings are insightful because AT2 cells are known to contain an elegant quality control network that responds to stress, internal or external," said Ghosh. "Failure of quality control leads to broader organ dysfunction and, in this case, fibrotic remodeling of the lung."
To conduct their study, Ghosh collaborated with co-senior author Debashis Sahoo, Ph.D., associate professor in the departments of Computer Science, Engineering and Pediatrics at UC San Diego to access transdisciplinary approaches, such as AI-assisted "big data" analysis.
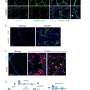
Ghosh and Sahoo said the approach would help them stay unbiased in navigating the unknowns of an emerging, post-pandemic disease. They analyzed more than 1,000 human lung transcriptomic datasets associated with various lung conditions, specifically looking for gene expression patterns, inflammation signaling and cellular changes. The disease with the closest match: IPF.
The authors were able to successfully induce these tell-tale elements in human lung organoids, in a hamster model of COVID-19, and could confirm their presence in the lungs of deceased individuals with COVID-19. Key elements were also reversed in the hamsters using anti-SARS-COV-2 therapeutics. A deeper analysis pinpointed endoplasmic reticulum stress as the shared early trigger of both post-COVID lung disease and ILD.
Ghosh said the use of computational models to identify shared gene expression and cellular processes between COVID-19 and IPF suggests utility of our findings beyond the current pandemic.
"The insights, biomarkers, tools, mechanisms and promising therapeutic avenues identified here are likely to spur the development of therapies for patients with IPF and other fibrotic interstitial lung diseases, all of whom have limited or no treatment options."
IPF affects approximately 100,000 persons in the United States, with 30,000 to 40,000 new cases annually. The condition has a poor prognosis, with an estimated mean survival of 2 to 5 years from time of diagnosis.
More information: Saptarshi Sinha et al, COVID-19 lung disease shares driver AT2 cytopathic features with Idiopathic pulmonary fibrosis, eBioMedicine (2022). DOI: 10.1016/j.ebiom.2022.104185