Deliberately damaging DNA could boost the effectiveness of immunotherapy in kidney cancer
DNA damage is one of the foundational causes of cancer. But researchers have now found that deliberately causing DNA damage—by delivering additional treatments like radiotherapy—could improve the effectiveness of immunotherapy for some people with kidney cancer.
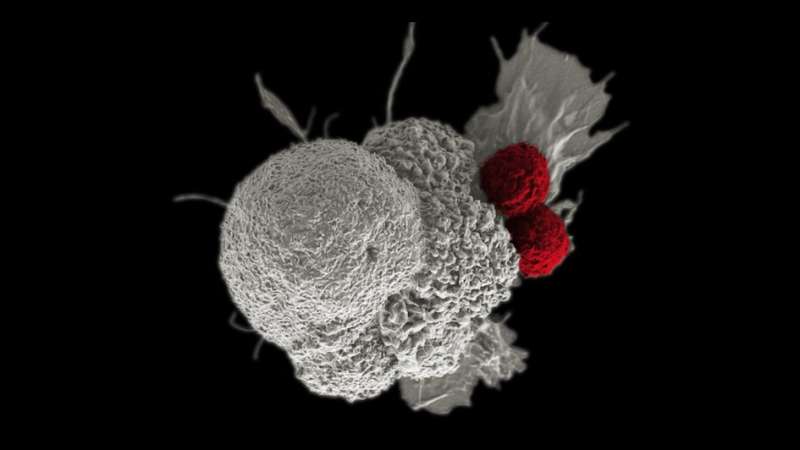
Immunotherapy can work by stimulating people's own immune systems to fight cancer more effectively. In people with kidney cancer who also have a mutation in the PBRM1 gene, damaging DNA before immunotherapy could further activate the immune system.
The study, led by scientists at The Institute of Cancer Research, London, and published in Genes and Development, sheds light on the role of PBRM1, and suggests that kidney cancer patients with mutations in the gene could benefit from a combined treatment of radiotherapy and immunotherapy.
Findings suggest that the PBRM1 gene is crucial in preventing cells from dividing when they contain damaged DNA. Researchers found that mutations in PBRM1 disrupt this process and allow the cells with damaged DNA to multiply, triggering inflammatory signals that are thought to enhance the patient's immune response against cancer.
Mutation of the PBRM1 gene
The PBRM1 gene is commonly mutated in clear cell renal cancer, a type of kidney cancer, with mutations that cause loss of function found in approximately 40% of cases. When functioning normally, it plays an important role in cell division—more precisely in the "G2/M checkpoint," a step of cell division which prevents cells with damaged DNA from dividing until the damage is repaired.
When scientists "switched off" the PBRM1 gene in the lab, they found that this checkpoint could be initiated but not maintained, resulting in the premature division, and spread of cells with DNA damage.
Innate immune signaling is the body's natural response to injury or infection. When cells with DNA damage are allowed to multiply, they trigger these signaling pathways, alerting the immune system to the detected threat and causing nearby cells to heighten their anti-tumor defenses.
By switching the PBRM1 gene back on, scientists found they were able to stop the cells with damaged DNA from multiplying, confirming that the PBRM1 gene is crucial for proper function of the G2/M checkpoint.
A new approach to treatment
The idea of combining DNA-damaging therapies with immunotherapy is already starting to be introduced in both the laboratory and clinic. However, in-depth understanding of its relationship with the PBRM1 gene opens up new possibilities for treating kidney cancer.
Radiotherapy is not typically used to treat kidney cancer, which tends to be resistant to radiation. But instead of killing the cells directly, scientists suggest that the DNA damage caused by radiotherapy could set off the inflammatory signaling pathway, increasing the effect of immunotherapy for those with PBRM1 mutation.
Predicting immunotherapy response
Scientists have previously investigated whether PBRM1 mutation could be used to predict immunotherapy response, but while some studies showed that the mutation was associated with improved outcomes, others produced contradictory results.
This research provides new insight into the precise biological pathways through which defects in the PBRM1 gene influence immunotherapy outcomes. It also suggests that the inconsistent results from previous studies could be explained by natural differences in patients' immune signaling pathways.
Responses to immunotherapy treatment can vary widely, so it is important to develop new ways of identifying patients who are most likely to benefit. Understanding these mechanisms and markers is important for personalizing treatment approaches, while also sparing patients who are unlikely to benefit from immunotherapy the side effects of treatment.
Serendipity in discovery science
Study lead Professor Jessica Downs, Deputy Head of the Division of Cancer Biology and Leader of the Epigenetics and Genome Stability Team at the ICR, said, "We weren't setting out to improve therapy or look for a biomarker when we started this study, so our findings highlight the importance of discovery science, which seeks to understand how things work at the basic level. We were simply characterizing cells when we stumbled across observations that could have real therapeutic value.
"The research is still in its early stages, and we still need to test if combining radiotherapy and immunotherapy works effectively in animal models of kidney cancer before we can begin to think about clinical trials.
"Around 120,000 patients a year are diagnosed with renal cancer with loss of PBRM1 worldwide. And fewer than a quarter of those patients will respond well to immunotherapy. Our findings are something that could really benefit a large group of people that don't have many options."
Dr. Henry Stennett, research information manager at Cancer Research U.K., said, "This is an excellent example of the power of discovery science, which is at the heart of what we do. By digging into the detail of how this important gene works, our researchers have transformed our understanding of the relationship between DNA damage and kidney cancer. These results could soon lead to more effective treatments for people with the disease."
More information: Hugang Feng et al, PBAF loss leads to DNA damage-induced inflammatory signaling through defective G2/M checkpoint maintenance, Genes & Development (2022). DOI: 10.1101/gad.349249.121