COVID-19 vaccination shown to protect people with blood cancer
People suffering from blood cancer often have a weak immune system, putting them at higher risk of falling seriously ill with COVID-19. Some cancer therapies, moreover, result in these patients forming few or no antibodies against SARS-CoV-2 after COVID-19 vaccination. However, vaccination can also activate so-called T cells, which are responsible particularly for the long-term immune response.
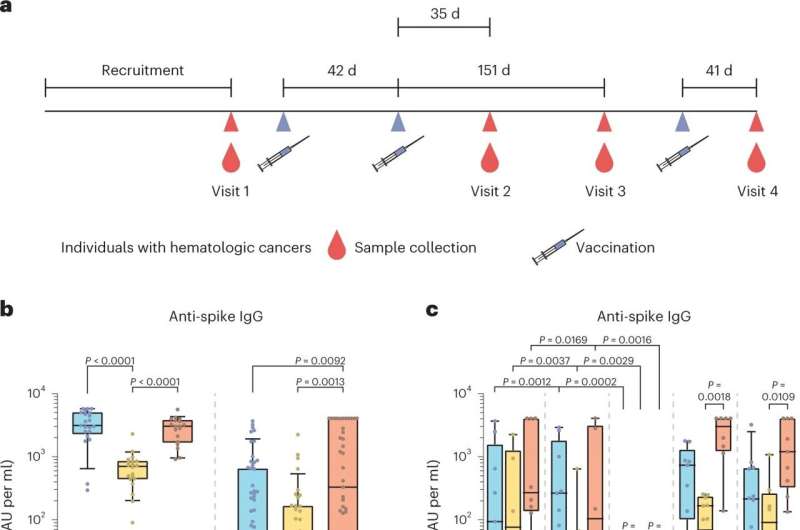
A team led by physicians Dr. Andrea Keppler-Hafkemeyer and Dr. Christine Greil from the Medical Center-University of Freiburgand virologist Prof. Oliver T. Keppler from LMU Munich has now characterized in detail the course over several months of the immune response of patients with blood cancer who had received a total of three vaccinations against COVID-19. The results allow inferences to be made about the protection that vaccination gives these patients against serious illness from SARS-CoV2.
Strong T cell response to COVID-19 vaccination
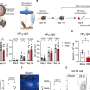
The study focused on patients with two kinds of blood cancer: B-cell lymphoma and multiple myeloma. "Our results show that almost all study participants had a strong T cell response to COVID-19 vaccination," explains Dr. Andrea Keppler-Hafkemeyer.
"This could be one reason why breakthrough infections turned out to be mild to moderately severe even in study participants who had been unable to form any specific antibodies after vaccination because of their therapy," adds Dr. Christine Greil. The co-principal investigators and lead authors regularly look after blood cancer patients in the Department of Medicine I at the Medical Center—University of Freiburg.
The research group led by Prof. Oliver T. Keppler is specialized not only in analyzing the concentration of antibodies after vaccination, but also their quality. This depends particularly on the strength of the bonds between antibodies and the viral spike protein. In addition, the ability of antibodies to neutralize different SARS-CoV-2 variants in cell cultures plays a major role.
As the next step, therefore, the scientists compared the quantity and quality of antibodies and T cell responses to the spike protein among blood cancer patients and healthy study participants after two and three COVID-19 vaccinations.
High-quality antibodies against different SARS-CoV-2 variants
The study revealed that patients who can form antibodies tend to produce antibodies of particularly high quality. After their second vaccination, they are already able to neutralize and thus deactivate different SARS-CoV-2 variants. This ability is considerably more pronounced in this patient cohort than in vaccinated healthy people.
"COVID-19 vaccination can generate very broad antiviral immunity—including highly potent neutralizing antibodies—in patients with various types of blood cancer. Consequently, multiple vaccine doses can be recommended for patients with B-cell lymphoma or multiple myeloma without interrupting therapy," summarizes Prof. Oliver T. Keppler.
The paper is published in the journal Nature Cancer.
More information: Andrea Keppler-Hafkemeyer et al, Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma, Nature Cancer (2022). DOI: 10.1038/s43018-022-00502-x