This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Brain tumor clinical trial: Failed Alzheimer's drug that boosts effect of radiotherapy to be tested in humans this year
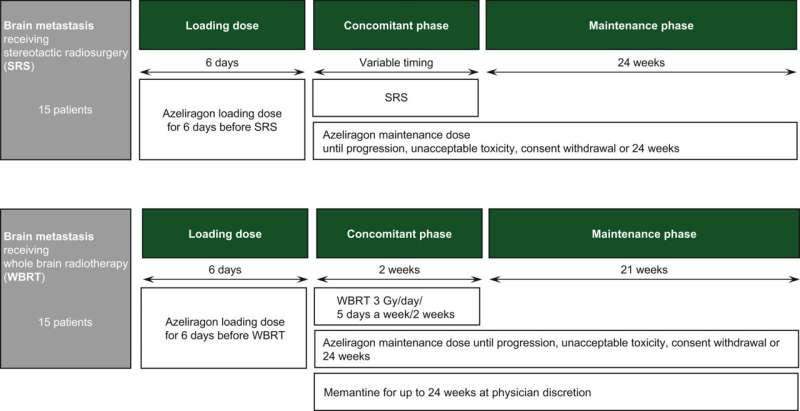
Cancer researchers in Spain are set to launch a clinical trial this year testing a new combination radiotherapy treatment for brain tumors. A combination of chemoradiotherapy and azeliragon, a drug working as a RAGE inhibitor, will be tested in newly diagnosed glioblastoma patients in a new phase I/II clinical trial based in Spain. Dr. Manuel Valiente and his team at the Spanish National Cancer Research Centre (CNIO) have published their plans for the trial today in ESMO Open.
Brain tumors can be difficult to treat, whether caused by brain cancers like glioblastoma or when cancer cells spread to the brain from elsewhere in the body. Sadly, they are the leading cause of cancer death in children and adults under 40 in the UK. Radiotherapy is a gold-standard treatment for brain tumors, however in many patients the tumor resists treatment and continues to grow.
This upcoming clinical trial hopes to boost the effect of radiotherapy on brain tumors in patients that otherwise would not respond to treatment. Azeliragon, a RAGE inhibitor, will be used in combination with chemoradiotherapy in the new trial to block the resistance response Dr. Valiente believes to be responsible for treatment resistance ("radioresistance") in many cases. The drug has previously been tested for use treating Alzheimer's disease—though it did not show the desired effect in that trial, it was found to be safe for use in humans.
Dr. Manuel Valiente, lead author of the study, said, "Brain tumors create many challenges when it comes to treatment. Radiotherapy as a cancer treatment has been one of the great successes of cancer research, but, from a molecular perspective, the way we administer it hasn't actually changed much in the last couple of decades, and we know that it doesn't work for many patients."
"We wanted to uncover why this is the case and find a way to help more patients with brain tumors, as often these patients find they have few to no options for treatment. We are excited to have found a new way to do just that."
"It is really amazing how quickly the results have translated to the clinic. We have identified the problem, found a biomarker to screen for the problem, and, crucially, a solution. Now, we will see if we can replicate this effect in human participants. I am hopeful that we could make progress in the trial quickly because we already know that azeliragon is safe to use in humans."
Dr. Lynn Turner, Director of Research at Worldwide Cancer Research, said, "Cutting-edge discovery research, like that carried out by Dr. Valiente, is essential if we hope to find brand new cures for cancer. We need to explore brand new avenues of research to find the big breakthroughs that will progress our understanding of cancer, and better ways to treat it. That is why this is the focus of our charity—you have to think outside of the box to discover big breakthroughs like this."
"Brilliant research like this is only made possible thanks to our Curestarters, a community of people focused on ending the suffering caused by cancer by starting new cancer cures. We are incredibly grateful for their efforts and generosity."
Crucially, a sister prospective study will follow 200 patients with cancer that has spread to the brain to see if the biomarker linked to radioresistance (S100A9) can predict which patients will respond to radiotherapy. If successful, and azeliragon is found to stop radioresistance, personalized treatment could help to enhance the effect of radiotherapy for patients with brain tumors—rather than administer a lengthy and ultimately ineffective treatment.
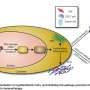
The trial and prospective study are based on a major breakthrough made by Dr. Manuel Valiente and his team last year. They discovered:
- a mechanism that stops radiotherapy from working against brain tumors in many cases,
- how to test for the mechanism using a new biomarker (S100A9),
- that a drug called a RAGE inhibitor could block this process and allow radiotherapy to destroy cancer cells.
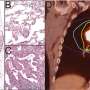
In their experiment, all seven samples from patients showing radioresistance responded to radiotherapy when a RAGE inhibitor was used simultaneously.
More information: M. Valiente et al, Emerging targets for cancer treatment: S100A9/RAGE, ESMO Open (2023). DOI: 10.1016/j.esmoop.2022.100751