This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Investigational drug may combat brain tumors by targeting cancer cells' fat production
Because glioblastoma, a highly aggressive and lethal brain cancer, is resistant to conventional therapies, investigators are searching for characteristics of glioblastoma cells that could point to promising drug targets.
One such characteristic is the cells' reliance on what's called de novo lipid synthesis—or the conversion of carbohydrates to fats—to support the cells' energy demands.
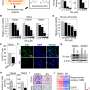
New research led by scientists at Massachusetts General Hospital (MGH) reveals that a drug that inhibits the enzyme stearoyl CoA Desaturase 1 (SCD) interferes with this process, and when administered to mice with glioblastoma, the drug delays tumor growth and increases glioblastoma cells' sensitivity to anticancer therapies. The findings, which are published in Science Translational Medicine, may lead to new treatment options for patients.
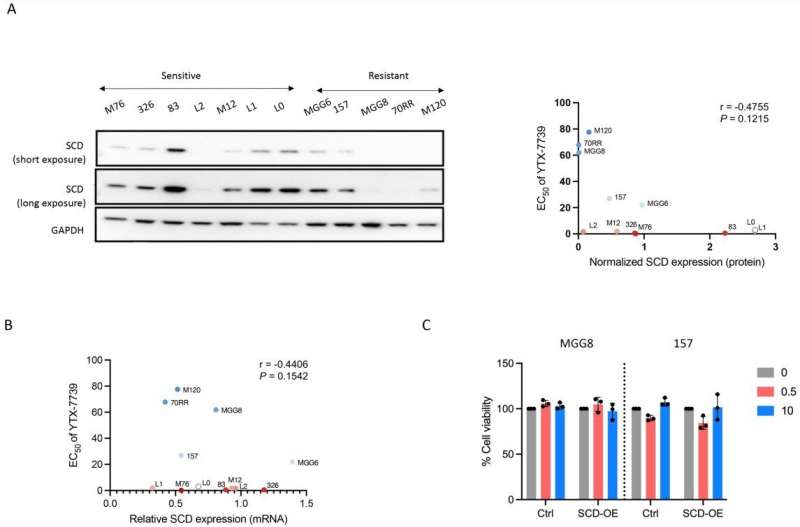
During one step of de novo lipid synthesis, SCD converts saturated fatty acids to monounsaturated fatty acids. Previously, Christian Badr, Ph.D., an assistant in Neuroscience at MGH and an assistant professor of Neurology at Harvard Medical School, and his colleagues showed that glioblastoma cells depend on activation of SCD and the availability of monounsaturated fatty acids.
In this new research, the team tested the anti-glioblastoma potential of an SCD inhibitor, YTX-7739, that can cross the blood brain barrier and is being evaluated as an oral drug in phase I clinical trials for the treatment of patients with Parkinson's disease.
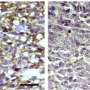
The investigators found that YTX-7739 was toxic to patient-derived glioblastoma stem cells. By blocking SCD, the cells accumulated too many saturated fatty acids, a process referred to as lipotoxicity. Also, when administered to mice with tumors, YTX-7739 inhibited processes involved in fatty acid metabolism in glioblastoma cells and increased the cells' sensitivity to conventional glioblastoma chemotherapy.
When examining the detailed mechanisms behind YTX-7739's effects on cells, the scientists found that the MEK/ERK signaling pathway renders glioblastoma cells particularly vulnerable to YTX-7739, whereas the AMPK signaling pathway acts to protect glioblastoma cells and can make them resistant to the loss of de novo lipid synthesis that occurs when YTX-7739 is present.
"Based on our results, we propose that MEK/ERK and AMPK activities, which can be detected in tumor biopsies, could be predictive biomarkers to guide patient selection and stratification," says Badr.
In other words, patients whose tumors have robust MEK/ERK activity would likely benefit from therapies such as YTX-7739, whereas those with high AMPK activity likely would not. "Our findings should also help tailor treatment paradigms to maximize therapeutic efficacy.
For instance, some widely used drugs, such as the anti-inflammatory agent salicylate or the anti-diabetic compound metformin, are potent activators of AMPK and could be detrimental to the efficacy of YTX-7739 or other de novo lipid synthesis–targeting therapies," says Badr.
More information: Katharina M. Eyme et al, Targeting de novo lipid synthesis induces lipotoxicity and impairs DNA damage repair in glioblastoma mouse models, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abq6288