This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Movement shown to reduce sensory responses in Parkinson's disease
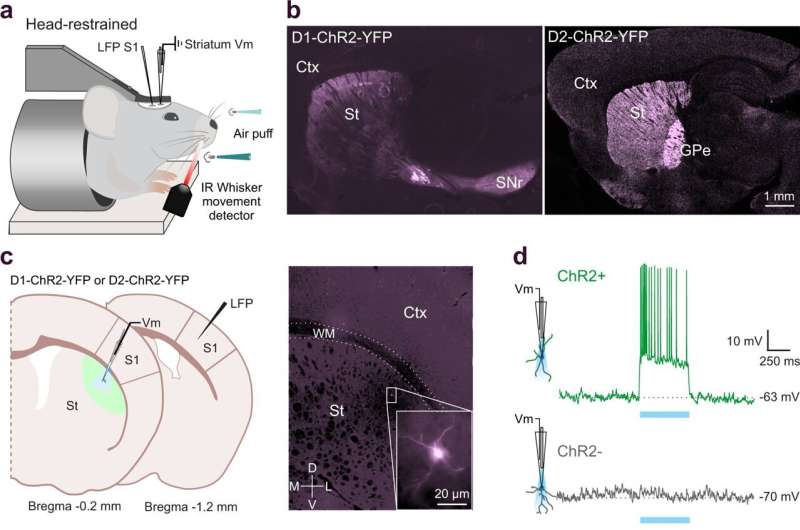
Researchers at Karolinska Institutet have been looking at how movement shapes our senses and how sensory and motor processes are both affected in Parkinson's disease. In a study published in Nature Communications, de la Torre-Martinez et al, show that movement reduces the responses to sensory input and that both sensory and motor processes are altered in an animal model of Parkinson's disease.
Movement control and sensory integration are the fundamental functions of our nervous system. Within the brain, a group of deep nuclei called the basal ganglia are involved in such sensorimotor functions. The basal ganglia are also the primary brain region affected in Parkinson's disease.
"The basal ganglia are involved in both sensory and motor functions and we show here how movement shapes sensory responses," says Gilad Silberberg, professor at the Department of Neuroscience at Karolinska Institutet and corresponding author of the article. "We also show that in Parkinsonian mice both sensory integration and movement-related responses are changed."
Most previous work in the basal ganglia field focused on motor impairments, while here, the researchers highlight the interactions between motor and sensory functions.
"The results are important for our understanding of sensory-motor interactions in normal brain function. We show that single neurons in the basal ganglia respond to both sensory stimulation and movement. We also show that movement reduces the strength of sensory inputs and we describe the mechanisms that make it happen," says Roberto de la Torre-Martinez, postdoctoral researcher and co-author of the study.
Electrical recording of deep brain neurons
The researchers used a special electrical recording method called patch-clamp electrophysiology, which received the Nobel Prize in 1991.
"Here, we used this method to record deep brain neurons in awake mice while they integrated sensory inputs. These recordings are technically challenging but they can provide information about the synaptic inputs to neurons while the animal is engaged in sensory-motor functions. We used healthy mice and compared their sensory-motor properties with Parkinsonian (dopamine-depleted) mice," explains Maya Ketzef, assistant professor at the same department and co-author of the study.
The next step is to combine the patch-clamp recordings with imaging of different neuromodulators in the basal ganglia.
"Traditionally, dopamine has been the main neuromodulator studied in the basal ganglia, and we would like to study how dopamine interacts with other neuromodulators such as acetylcholine and serotonin," says Gilad Silberberg.
More information: Roberto de la Torre-Martinez et al, Ongoing movement controls sensory integration in the dorsolateral striatum, Nature Communications (2023). DOI: 10.1038/s41467-023-36648-0