This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Recommendations on using plasma EBV DNA in clinical management of nasopharyngeal carcinoma
Led by the Department of Clinical Oncology, School of Clinical Medicine, LKS Faculty of Medicine, the University of Hong Kong (HKUMed), an international group of the world's top clinicians in managing nasopharyngeal carcinoma (NPC) has recently published recommendations on measuring plasma EBV DNA in managing NPC in an acute setting of serious resource and personnel constraints during the COVID-19 pandemic.
The international recommendations serve as important guidelines on future management of NPC when severe disasters or disruptions arise in future. The consensus statements of these recommendations were published in the oncology research journal The Lancet Oncology.
NPC is endemic in Southern China, Southeast Asia, and North and East Africa, which is highly associated with prior Epstein-Barr virus (EBV) infection. It is also common in Hong Kong, closely related to genetic predisposition and dietary factors such as consumption of Chinese-style salted fish, with an age-standardized incidence rate of 5.8 per 100,000 population in 2020, ranked 8th and 17th for males and females respectively.
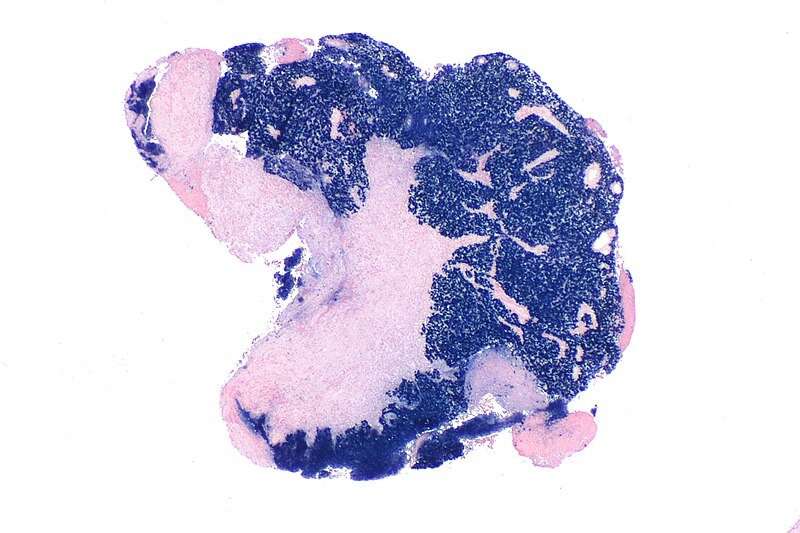
While histological proof is the gold standard in NPC diagnosis, plasma EBV DNA, the secretory DNA fragments of EBV, easily extracted in routine blood taking, has been regarded as the most sensitive and accurate tumor marker that has gained wide popularity and acceptance in NPC screening, diagnosis, stage segregation, treatment response monitoring, and prognostication.
Currently, standard investigations in clinical management of NPC include clinical examinations, imaging scans and nasoendoscopy, a high-risk procedure of aerosol generation. However, the essential personnel, facilities and resources could be exceedingly scarce and constrained during the raging COVID-19 pandemic. Therefore, it is sensible to consider whether plasma EBV DNA could replace nasoendoscopy, imaging scans, and even face-to-face clinical consultations during the COVID-19 pandemic and in the future when similar or even more devastating pandemics occur, leading to insufficiency of personnel and personal protective equipment.
The Department of Clinical Oncology invited 33 top international clinicians from a broad range of clinical disciplines (head and neck surgery or otorhinolaryngology, medical oncology, radiation oncology and clinical oncology), representing 51 international professional societies and national clinical trial groups across four continents (Asia, Africa, Europe, and North America) during the COVID-19 pandemic to complete a modified Delphi consensus process of three rounds with 22 questions on whether plasma EBV DNA can replace the aforesaid routine clinical procedures and consultations in various clinical settings of NPC management when they are substantially restrained.
In particular, all experts (100%) disagreed that the use of plasma EBV DNA and IgA viral capsid antigen, without imaging tools, could replace nasoendoscopy and biopsy as the only staging investigations for NPC in the resource-constrained setting. Even with the availability of imaging tools, 73% of the experts disagreed that the use of plasma EBV DNA should replace nasoendoscopy and biopsy as the only staging investigation for NPC.
The findings revealed that although plasma EBV DNA (supplemented, when possible, by imaging scans) can be used in most clinical circumstances in the context of resource limitations, it cannot entirely replace nasoendoscopy and tumor biopsy. All experts who rejected the use of plasma EBV DNA as the only tool in NPC management maintained that these procedures are essential, and that the patients' right to receive these standard investigations should not be suppressed, insofar as the health-care system allows.
These recommendations have taken into consideration various clinical scenarios from the joint perspectives of surgeons and non-surgeons, unlike previous recommendations published by individual clinical disciplines separately.
Significance of the consensus
In contrast to previous recommendations that endoscopic examinations should be exclusive to patients who are deemed to be at high risk of cancer recurrence and mortality from head and neck cancers and only when adequate personal protective equipment is available, this latest consensus concludes that merely measuring plasma EBV DNA should not replace the conventional but essential nasoendoscopic examinations, even when imaging services are very limited. Plasma EBV DNA combined with imaging scans can be considered an acceptable alternative in a very restricted clinical setting, but cannot replace face-to-face consultations and nasoendoscopy, and it cannot be used alone without imaging in NPC management.
Dr. Victor Lee Ho-fun, chairperson and clinical associate professor, Department of Clinical Oncology, School of Clinical Medicine, HKUMed, who spearheaded and designed the survey, commented, "These recommendations, which are the results of concerted efforts of international collaboration and cooperation, can serve as references and be used in other acute settings or during natural disasters, where there is an unexpectedly high risk of health hazards to patients and health care workers and a severe paucity of health personnel and resources."
Dr. Lee also hopes that "these recommendations can further stimulate international harmonization of plasma EBV DNA leading to more affordable use at institutions and hospitals in low-income and middle-income countries, and foster future researches in devising more sensitive and accurate tumor markers for NPC and other cancers when the pandemic is much contained."
More information: Victor Ho-Fun Lee et al, International recommendations for plasma Epstein-Barr virus DNA measurement in nasopharyngeal carcinoma in resource-constrained settings: lessons from the COVID-19 pandemic, The Lancet Oncology (2022). DOI: 10.1016/S1470-2045(22)00505-8