This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Terminal sterilization of oligonucleotide drug products
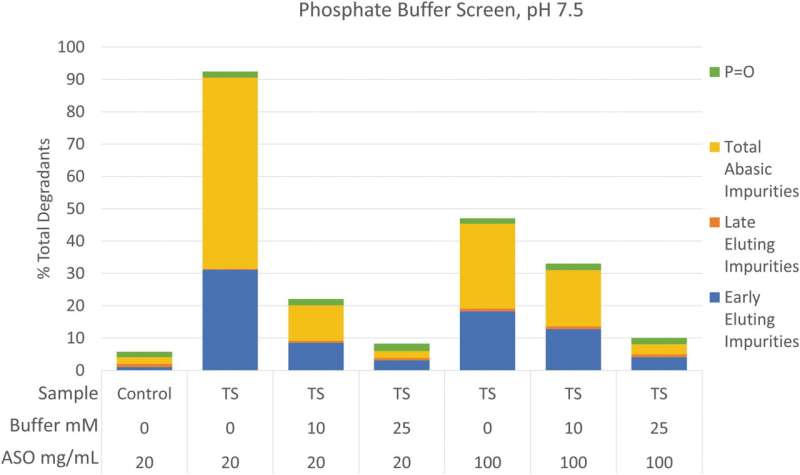
A new report, coauthored by several major pharmaceutical companies, reviews the current state of sterile oligonucleotide drug product processing. The article, which provides recommendations to aid in the evaluation and development of terminal sterilization processes, is published in Nucleic Acid Therapeutics.
All marketed oligonucleotide products are delivered as sterile preparations for parenteral delivery. The two most common methods for sterilizing parenteral drug products are terminal sterilization and membrane sterilization. Terminal sterilization provides greater sterility assurance than membrane sterilization, but not all drug products are amenable to terminal sterilization, which usually involves exposure to high heat or ionizing radiation.
Regarding the terminal sterilization of oligonucleotides, the authors provide recommendations for formulation development, assessing changes in the purity and impurity profile after terminal sterilization, and selecting the correct container closure.
"As the field continues to advance, commercial and academic researchers alike look to Nucleic Acid Therapeutics to provide the community with clear, detailed guidance on candidate therapeutic development and processing," says Executive Editor Graham C. Parker, Ph.D., Department of Pediatrics, Wayne State University School of Medicine, Detroit, MI.
More information: Daniel Paul DeCollibus et al, Considerations for the Terminal Sterilization of Oligonucleotide Drug Products, Nucleic Acid Therapeutics (2023). DOI: 10.1089/nat.2022.0073