This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
US FDA approves nasal spray for migraines

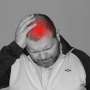
The US Food and Drug Administration has approved a fast-acting nasal spray from Pfizer designed to treat migraines, the US pharmaceutical giant said Friday.
Pfizer said it expected the drug, marketed under the name Zavzpret, to be available in pharmacies in July 2023.
"The FDA approval of Zavzpret marks a significant breakthrough for people with migraine who need freedom from pain and prefer alternative options to oral medications," Pfizer chief commercial officer Angela Hwang said in a statement.
A Phase 3 study of the drug found that it delivered pain relief to some migraine sufferers in as little as 15 minutes.
"As a nasal spray with rapid drug absorption, Zavzpret offers an alternative treatment option for people who need pain relief or cannot take oral medications due to nausea or vomiting," Pfizer quoted Kathleen Mullin, associate medical director at the New England Institute for Neurology and Headache, as saying.
The treatment for a condition generally tackled with orally taken medicines was double-blind tested on a sample of 1,405 people, with half taking a single spray dose and the remainder receiving a placebo.
The spray was found to reduce pain significantly when assessed two hours after the onset of a migraine, which as well as causing often severe headaches can include nausea and sensitivity to light or noise.
Pfizer acquired Zavzpret, also known as Zavegepant, last year for some $10 billion from Biohaven, along with other migraine treatments from the firm.
Some 39 million Americans experience migraine headaches, according to the American Migraine Foundation.
© 2023 AFP