This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Tracking the cellular and genetic roots of neuropsychiatric disease
A new analysis has revealed detailed information about genetic variation in brain cells that could open new avenues for the targeted treatment of diseases such as schizophrenia and Alzheimer's disease.
The findings, reported May 23 in Science, were the result of a multi-institutional collaboration known as PsychENCODE, founded in 2015 by the National Institutes of Health, which seeks new understandings of genomic influences on neuropsychiatric disease. The study was published alongside related studies in Science, Science Advances, and Science Translational Medicine.
Previous research has established a strong link between a person's genetics and their likelihood of developing neuropsychiatric disease, says Mark Gerstein, the Albert L. Williams Professor of Biomedical Informatics at Yale School of Medicine and senior author of the new study.
"The correlations between genetics and your susceptibility to disease are much higher for brain diseases than for cancer or heart disease," said Gerstein. "If your parents have schizophrenia, you're much more likely to get it than you are to get heart disease if your parents have the disease. There is a very large heritability for these brain-related conditions."
What's less clear, however, is how this genetic variation leads to disease.
"We want to understand the mechanism," said Gerstein. "What is that gene variant doing in the brain?"
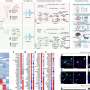
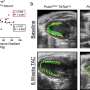
For the new study, researchers set out to better understand the genetic variation across individual cell types in the brain. To do so, they performed several types of single-cell experiments on more than 2.8 million cells taken from the brains of 388 people, including healthy individuals and others with schizophrenia, bipolar disorder, autism spectrum disorder, post-traumatic stress disorder, and Alzheimer's disease.
From that pool of cells, the researchers identified 28 different cell types. Then they examined gene expression and regulation within those cell types.
In one analysis, the researchers were able to link gene expression to variants in "upstream" regulatory regions, bits of genetic code situated before the gene in question that can increase or decrease the gene's expression.
"That's useful because if you have a variant of interest, you can now link it to a gene," said Gerstein. "And that's really powerful because it helps you interpret the variants. It helps you understand what effect they're having in the brain. And because we looked across cell types, our data also allow you to connect that variant to an individual cell type of action."
The researchers also assessed how particular genes, such as those associated with neurotransmitters, varied across individuals and cell types, finding variability was usually higher across cell types than across individuals. This pattern was even stronger for genes that code for proteins targeted for drug treatment.
"And that's generally good for a drug," Gerstein said. "It means that those drugs are homing in on particular cell types and not affecting your whole brain or body. It also means those drugs are more likely to be unaffected by genetic variants and work in many people."
Using the data generated by the analysis, the researchers were able to map out within-cell type genetic regulatory networks and between-cell communication networks, and then plug those networks into a machine learning model. Then, using an individual's genetic information, the model could predict whether they had a brain disease.
"Because these networks were hard coded in the model, when the model made a prediction we could see which parts of the network contributed to it," said Gerstein. "So we could identify which genes and cell types were important for that prediction. And that can suggest candidate drug targets."
In one example, the model predicted an individual with a particular genetic variant might have bipolar disorder, and the researchers could see that prediction was based on two genes in three cell types. In another, the researchers identified six genes in six cell types that contributed to a schizophrenia prediction.
The model also worked in the opposite direction. The researchers could introduce a genetic perturbation and see how that might affect the network and an individual's health. This, Gerstein says, is useful for drug design or previewing how well drugs or drug combinations might fare as treatments.
Together, the findings could help facilitate precision-medicine approaches for neuropsychiatric disease, said the researchers.
To further this work, the consortium has made its results and model available to other researchers.
"Our vision is that researchers interested in a particular gene or variant can use our resources to better understand what it's doing in the brain or to perhaps identify new candidate drug targets to investigate more," said Gerstein.
More information: Prashant Emani et al, Single-cell genomics and regulatory networks for 388 human brains, Science (2024). DOI: 10.1126/science.adi5199. www.science.org/doi/10.1126/science.adi5199