This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Immune checkpoint inhibitor antitumor response: Decoding molecular mechanisms
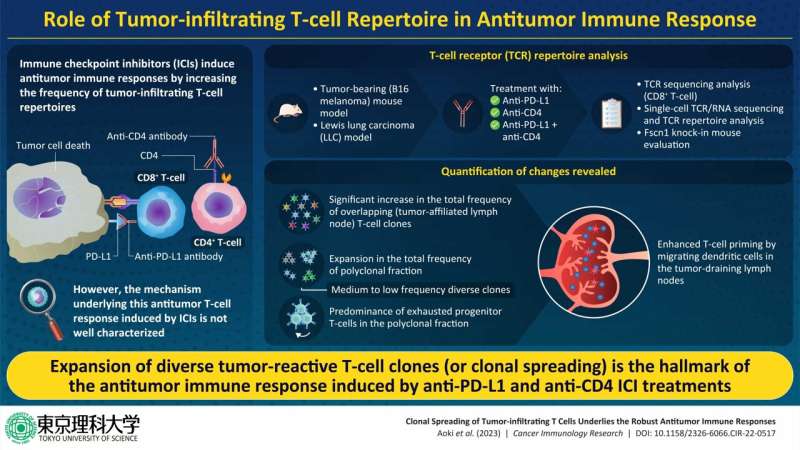
Immune checkpoint inhibitors (ICIs) are widely sought after for the treatment of different types of cancers. Unfortunately, only 20%–30% patients with cancer respond to ICI treatment. Although the factors that influence the positive or negative response to ICI treatment are poorly understood, the strength of the ICIs' antitumor response by TILs is thought to play a key role. Hence, investigating the antitumor response induced by ICIs might provide insights into their underlying mechanisms.
It is known that CD8+ tumor-infiltrating T-lymphocytes (TILs) are the primary effector cells that lead to antitumor response. TILs are composed of various T-cell clones that are determined by their unique T-cell receptor (TCR) genes. A diverse TCR repertoire can indicate the existence of TIL population that can recognize multiple tumor-antigens. However, the way that the diversity of tumor-reactive clones influences their antitumor effects remains unclear.
To this end, a team of researchers led by Associate professor Satoshi Ueha, including Dr. Shigeyuki Shichino and Professor Kouji Matsushima from the Tokyo University of Science, and Mr. Hiroyasu Aoki from the University of Tokyo, Japan, investigated the correlation between TIL clones and their antitumor effects. Their work was published in Cancer Immunology Research.
Discussing the team's motivation behind this study, Prof. Ueha explains, "By clarifying the mechanism by which ICIs increase tumor reactive CD8+ T cells, we hoped to develop a diagnostic method to predict the efficacy of this therapy as well as a new combined cancer immunotherapy."
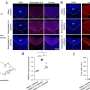
First, the team analyzed the TCR repertoire of B16F10 and Lewis lung carcinoma (LLC) tumor bearing mice, treated with the ICIs—anti-PD-L1 anti-CD4, and a combination of both (CD4 + PDL1) antibodies. They observed that the antibodies increased the mobilization of overlapping (OL) T-cell clones in draining lymph nodes (dLNs) and tumors.
Next, they categorized the dLN-tumor OL clones into oligoclonal and polyclonal fractions. They found that ICI administration significantly increased the polyclonal fraction of OL repertoire but not the oligoclonal fraction in both tumor model. These findings confirmed that the increase in dLN-tumor repertoire was due to clonal spreading, i.e., the expansion of diverse polyclonal clones, and not clonal skewing, i.e., the expansion of oligoclonal clones.
The team further found that increase of the polyclonal fraction of OL repertoire due to ICI administration was correlated with the tumor volume after treatment, a parameter reflecting an antitumor effect. Moreover, single-cell RNA and TCR sequencing exhibited that the polyclonal dLN-tumor OL clones were rich in progenitor exhausted T cells (T cells having more proliferative potential and play an important role in the antitumor effects of ICI).
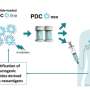
After establishing that clonal spreading was responsible for effective antitumor response to ICIs, Associate Prof. Ueha and his team investigated the mechanism of clonal spreading. They used tumor-bearing knock-in mice that were selectively depleted of dLN migratory dendritic cells (migDCs) and treated them with anti-PD-L1 and anti-CD4 antibodies. Upon examining the dLN-tumor OL clones, they noted that ICIs enhanced the priming of polyclonal tumor-reactive T-cells by migDCs in the dLN, resulting in clonal spreading.
So, what are the future implications of these findings? "Our results can be used to predict and diagnose the efficacy of ICIs, thereby making it possible to deliver these rather expensive drugs to patients who can benefit from them. In the long term, the clonal spreading response found in our study may be used to develop therapeutic drugs that induce stronger anti-tumor T-cell responses when used in combination with ICIs," says Prof. Ueha.
He also believes that focusing on a greater variety of tumor reactive clones and their proliferation can have important implications in advancing this field of research. "We are confident that the applications of these ICIs will soon extend to clinical trials and bring much needed respite to patients grappling with cancer."
More information: Clonal spreading of tumor-infiltrating T cells underlies the robust antitumor immune responses, Cancer Immunology Research (2023). DOI: 10.1158/2326-6066.CIR-22-0517