March 24, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Mitochondrial transplantation improves rat recovery from cardiac arrest
When a heart stops beating, blood stops flowing and delivering oxygen to the brain (hypoxia) and other vital organs (ischemia). There is a small window (about 4 minutes) before the lack of blood flow begins to damage the brain. After 10 minutes, severe brain damage is expected. The sooner the heart can be restarted, the less the chances of severe brain injury.
Researchers at the Feinstein Institutes for Medical Research have tested a novel approach to increase survival rates, mitigate damage and accelerate repair in the ischemic brains of rats via mitochondrial transplantation. In a paper, "Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest," published in the journal BMC Medicine, the researchers detail the steps they took from in vitro lab to in vivo rat models in achieving a 91% survival rate—a 36% improvement over the control.
According to the endosymbiotic theory, mitochondria originated as a bacteria that "got swallowed" and forged a symbiotic relationship with the host cell, evolving into the mitochondria within the eukaryotic cells of complex lifeforms. Still, mitochondria have kept some of their ancient bacterial characteristics. They have double membranes like gram-negative bacteria and the ability to generate ATP with aerobic respiration—which requires oxygen—which is why our cells need oxygen deliveries from blood.
When blood stops flowing and oxygen delivery stops, mitochondria can no longer produce energy, and soon the cell is in danger of dying. If the blood has stopped flowing everywhere in the body the danger is everywhere, but nowhere more so than in the brain.
Recent research has shown some promise for mitochondria being able to assist in the repair of other mitochondria. Injured mitochondria are capable of self-repair and cellular protection through fission, fusion, and mitophagy. Recently described mechanisms of intercellular mitochondrial transfer have been followed up with mitochondrial transplantations and were shown to have protective effects in muscular tissues.
The Feinstein research team decided to test the effectiveness of transplantation in a cardiac arrest event with a specific focus on neuronal tissue health.
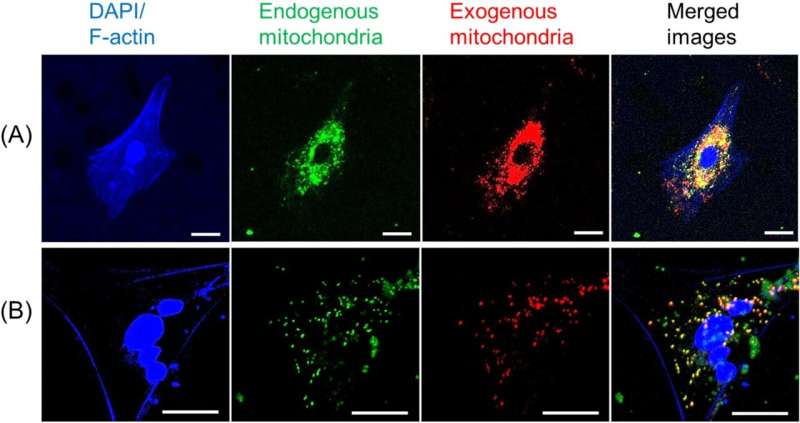
Before attempting to transfer mitochondria into rats, the researchers wanted to test if donor mitochondria can be taken up by neurons growing in culture. To do this, mitochondria extracted from rat brain and muscle tissues were stained red and mitochondria of the neural cells were stained green. The donated mitochondria were taken up into cultured neural cells and co-localized with endogenous mitochondria inside neural cells. With a successful lab test complete, it was time to try it in a live model.
Thirty-three rats were put into cardiac arrest for 10 minutes and then resuscitated and given one of three treatments: freshly isolated donor mitochondria, negative control solution, and nonfunctional (frozen/thawed) mitochondria to look for effects potentially due to adding similar amounts of mitochondrial building blocks (proteins, lipids, DNA, RNA) as the fresh sample.
In freshly isolated mitochondria transplantation the 72-hour survival rate was 91% compared to just 55% in the negative control. The excellent survival rates were associated with improvements in rapid recovery of arterial lactate, glucose levels, cerebral microcirculation, neurological function and decreased lung injury.
The study demonstrates the understudied potential for mitochondrial transplantation in tissue protection and recovery. A unique aspect of the study was the inclusion of nonfunctional frozen-thawed mitochondria as an additional control. The frozen-thawed mitochondria did not have a protective effect which strongly suggests that mitochondrial activity from the fresh samples is causational to the protective result.
A very intriguing finding came from gene expression measurements, suggesting fusion genes were significantly down-regulated during the recovery phase with the fresh transplantation group. With mitochondrial dynamics shifted toward fission, the current study seems at odds with some previous findings by other research in terms of expected outcomes. Which is just the sort of thing that future research will want to look into.
More information: Kei Hayashida et al, Exogenous mitochondrial transplantation improves survival and neurological outcomes after resuscitation from cardiac arrest, BMC Medicine (2023). DOI: 10.1186/s12916-023-02759-0
© 2023 Science X Network