This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers discover two-pronged approach to stimulate STING antitumor activity
Immunotherapies have greatly improved the outcomes of many patients with melanoma. But there is still a need for new approaches for the subset of patients who do not respond well to this type of therapy. Moffitt Cancer Center researchers are looking at new targets to help inhibit tumor development and promote antitumor immunity, one being the STING signaling pathway. In a new article published in Nature Communications, a team of Moffitt and University of Miami Miller School of Medicine investigators demonstrate that targeting the STING pathway with a combination strategy results in improved antitumor activity.
The STING pathway is a key regulator of immune responses to viruses and bacteria and contributes to antitumor immunity. Inhibition of STING signaling is observed in several cancer cell lines, and expression of STING protein decreases as some cancer types progress, such as melanoma. These observations suggest that agonist drugs, or drugs that activate STING signaling within the tumor environment, may have antitumor effects.
"This hypothesis has been confirmed in many pre-clinical laboratory studies. However, STING agonists have had disappointing results in human clinical trials, suggesting that an unknown mechanism is contributing to these poor responses," said lead study author Rana Falahat, Ph.D., a research scientist in the Immuno-Oncology Program at Moffitt. "This is what prompted our study."
The Moffitt and University of Miami team wanted to improve their understanding of STING signaling and investigate how to improve the antitumor activity of STING agonists. They previously demonstrated that the DNA region that controls STING protein expression is modified by a process called methylation, during which chemical methyl groups are added to the DNA. This modification reduces the protein level of STING, resulting in less STING protein to inhibit tumor development. The researchers hypothesized that drugs that block methylation and increase STING expression levels in tumor cells may work in conjunction with STING agonists to kill cancer cells.
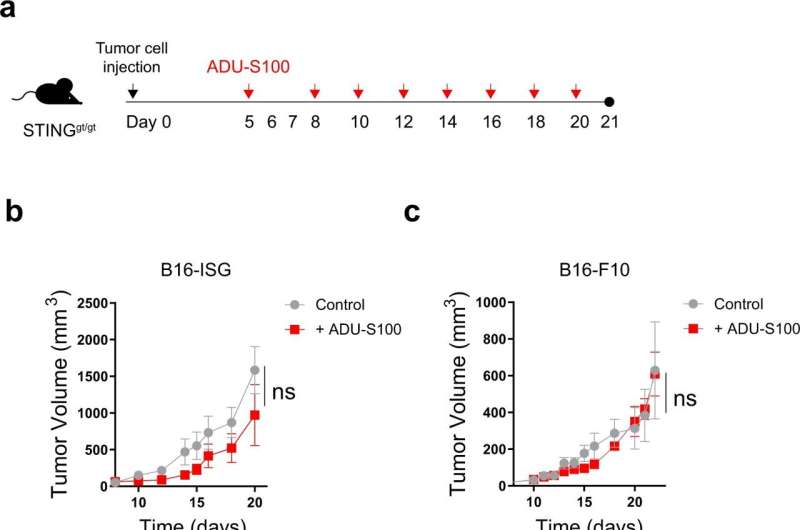
The researchers performed experiments in mouse models of melanoma with defective STING signaling either in the tumor cells or the surrounding immune cells. Similar to the human STING gene, they discovered that the mouse STING gene was also regulated by methylation. When the researchers treated cells with drugs that blocked methylation, STING expression and signaling increased, leading to the production of chemical messengers that enhanced antitumor immunity. They also demonstrated that treatment of mice with methylation inhibitors and STING agonists resulted in improved antitumor activity that was dependent on the presence and activity of immune cells called CD8 T cells.
These observations reveal that reactivating STING signaling in the tumor cells with methylation inhibitors can shape the therapeutic response to STING agonists. The researchers hope these preclinical findings will lead to better treatments for cancer patients in the clinic.
"Although additional work will be necessary to further identify optimal dosing levels and therapeutic schedules of each component, insights from our study provide a framework to design proper clinical treatment modalities with appropriate patient selection in melanoma and perhaps other solid tumors," said James J. Mulé, IPh.D., senior author, associate center director for Translational Science and the Michael McGillicuddy Endowed Chair for Melanoma Research and Treatment at Moffitt.
More information: Rana Falahat et al, Epigenetic state determines the in vivo efficacy of STING agonist therapy, Nature Communications (2023). DOI: 10.1038/s41467-023-37217-1