This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Action needed on breastfeeding data collection to gauge impact of medicines

A new systematic review from Swansea University in collaboration with ConcePTION, an Innovative Medicines Initiative (IMI), has called for action to monitor infants for any possible adverse drug reactions from exposure to medicines through breastmilk, even though problems may be rare; target additional support to breastfeeding patients whose medicines may affect breastfeeding; and provide long-term population-based data on any adverse events in infants exposed to medicines through breastmilk and any reduction in breastfeeding rates.
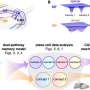
In the paper, which has been published in PLOS ONE, researchers identified only 10 established databases reporting on breastfeeding, medicines and infant outcomes: none reported education outcomes.
Researchers recommend that it should now be a priority that data on breastfeeding and medicines used by women during and after pregnancy and in labor are included in population databases along with data on subsequent long-term outcomes, including educational attainment, so that meaningful research can take place into the benefits and harms of medicines use during breastfeeding.
Professor Sue Jordan of the university's Faculty of Medicine, Health and Life Science, who led the research said, "The omission of breastfeeding data from most population databases indicates that there are few data to inform breastfeeding patients and those intending to breastfeed on whether lactation will be affected by prescription medicines, and how medicines will affect breastfed infants. To return investment in population healthcare databases pharmacoepidemiologists should have good quality data to explore any relationships between medicines exposures, breastfeeding and short- and long-term infant outcomes."
Review co-author, Dr. Sandra Lopez-Leon, an epidemiologist from Rutgers University and medicines company, Novartis said, "There is an urgent need to have high-quality linked data on medicines, long term childhood outcomes and modifiable risk factors, including breastfeeding so that robust analyses of medicine-related benefits and harms can take place, which will ultimately allow women to take informed decisions when making choices on their own medical treatment, and breastfeeding."
More information: Sue Jordan et al, Where are the data linking infant outcomes, breastfeeding and medicine exposure? A systematic scoping review, PLOS ONE (2023). DOI: 10.1371/journal.pone.0284128