This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Mechanism resembling aging and cancer found in a Finnish mitochondrial disease
A study has found that a mitochondrial disease in newborns shows cancer-like changes in proliferating cells, causing tissues to age prematurely. The finding is a significant step forward in understanding the syndrome and developing treatments for mitochondrial diseases.
GRACILE syndrome, a mitochondrial disease that is one of the Finnish heritage diseases, shows altered cell metabolism and proliferation resembling that of cancer cells. In the future, similar mitochondrial diseases could potentially be treated by limiting excessive cell proliferation. This is demonstrated in a study led by docent Jukka Kallijärvi and professor emerita Vineta Fellman that was carried out at the Folkhälsan Research Center and the University of Helsinki and published in Nature Communications in April 2023.
Mitochondria are organelles responsible for a large portion of cellular energy metabolism. Mutations in genes required for mitochondrial functions cause mitochondrial diseases in humans. GRACILE syndrome is caused by a malfunction in the respiratory chain, the very system the mitochondria utilize to generate cellular energy. The onset of the syndrome is in the fetal period, manifesting after birth as a liver and kidney disease with severe metabolic complications. Newborns with the syndrome usually only survive a few weeks.
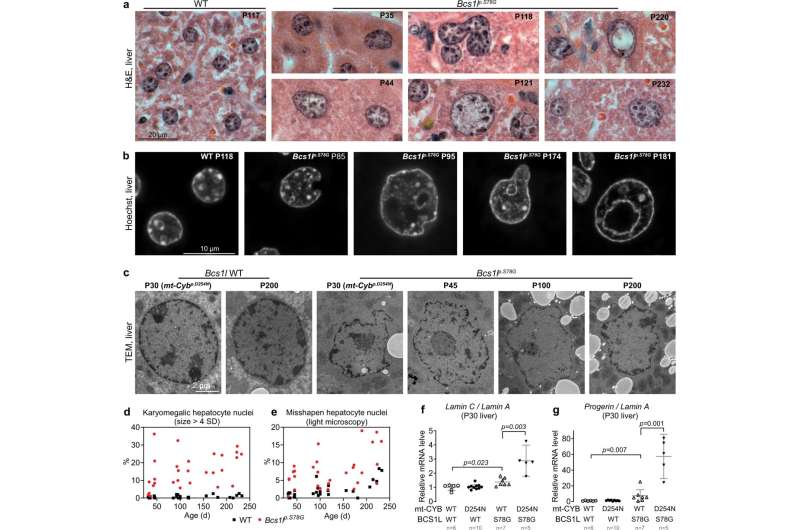
Using a mouse model, Kallijärvi's research group demonstrated that in the key tissues affected by the syndrome, cells accumulate massive DNA damage. The damage arises from the cells trying to grow and divide despite a lack of energy. Consequently, they fail the cell division cycle, and the tissues eventually drift into a state resembling premature aging.
Understanding the disease mechanism helps to develop therapies
Cell growth and replication of the genome consumes a lot of energy and building blocks, potentially making proliferating cells particularly susceptible to mitochondrial dysfunction. In multicellular organisms, evolution has developed strict mechanisms to safeguard the cell division process, for example as protection against cancer. However, some of these did not work in the mice carrying the GRACILE syndrome mutation.
The research group found that the expression of the cancer gene c-MYC had increased as much as 40-fold in the sick tissues. Inhibiting the function of c-MYC in the liver cells of the mutant mice with a miniprotein designed as a cancer drug reduced the DNA damage.
"This was the most astounding discovery in our study. A dramatic increase in a protein that promotes cell growth appears to force the cells to proliferate despite inadequate resources, resulting in a harmful vicious circle," Kallijärvi says.
Researchers prevented effects of aging in mice
Convincing evidence on illicit cell proliferation underlying premature aging came as a surprise in experiments where the researchers expressed a mitochondrial enzyme alternative oxidase as a kind of gene therapy to compensate for the dysfunction in the respiratory chain. Unexpectedly, AOX did not improve any of the main functions of the respiratory chain. Still, it almost completely suppressed mitochondrial stress signaling as well as the excessive cell proliferation, preventing the aging changes.
Interestingly, a ketogenic diet, which the researchers earlier found to improve the liver disease in these mice, had a similar effect on the cell cycle and DNA damage. Ketogenic diets have been trialed as a treatment in patients with milder mitochondrial diseases.
The researchers are now looking into which mechanisms triggered the mitochondrial stress signaling and whether blunting the harmful cell proliferation would alleviate the disease in the mouse model.
More information: Janne Purhonen et al, Mitochondrial complex III deficiency drives c-MYC overexpression and illicit cell cycle entry leading to senescence and segmental progeria, Nature Communications (2023). DOI: 10.1038/s41467-023-38027-1