This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The underlying mechanism and a potential therapeutic target for neuropathic pain
Neuropathic pain—abnormal hypersensitivity to stimuli—is associated with impaired quality of life and is often poorly managed. Estimates suggest that 3% to 17% of adults suffer from neuropathic pain, including a quarter of people with diabetes and a third of people with HIV.
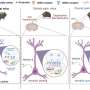
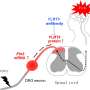
In a paper published in the journal Neuron, researchers report that a mechanism involving the enzyme Tiam1 in dorsal horn excitatory neurons of the spinal cord both initiates and maintains neuropathic pain. Moreover, they show that targeting spinal Tiam1 with anti-sense oligonucleotides injected into the cerebrospinal fluid effectively alleviated neuropathic pain hypersensitivity.
"Thus, our study has uncovered a pathophysiological mechanism that initiates, transitions and sustains neuropathic pain, and we have identified a promising therapeutic target for treating neuropathic pain with long-lasting consequences," said Lingyong Li, Ph.D., an associate professor at the University of Alabama at Birmingham Department of Anesthesiology and Perioperative Medicine. "Understanding the pathophysiological mechanisms underlying neuropathic pain is critical for developing new therapeutic strategies to treat chronic pain effectively."
Li and Kimberley Tolias, Ph.D., a professor at Baylor College of Medicine in Houston, Texas, were co-leaders of the research.
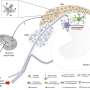
It was known that one feature of neuropathic pain is maladaptive changes in neurons of the spinal dorsal horn—increases in the size and density of dendritic spines, the primary postsynaptic sites of excitatory synapses. However, the mechanisms driving this synaptic plasticity were unclear. Dendrites are tree-like appendages attached to the body of a neuron that receive communications from other neurons. The spinal dorsal horn is one of the three gray columns of the spinal cord.
In related work, Li and Tolias last year found that chronic pain in a mouse model leads to an activated Tiam1 in anterior cingulate cortex pyramidal neurons of the brain, resulting in an increased number of spines on the neural dendrites. This higher spine density increased the number of connections, and the strength of those connections, between neurons, a change known as synaptic plasticity. Those increases caused hypersensitivity and were associated with chronic pain-related depression in the mouse model.
The current neuropathic pain study by Li and Tolias used mouse models of neuropathic pain caused by nerve injury, chemotherapy or diabetes. The researchers showed that Tiam1 is activated in the spinal dorsal horn of mice subjected to neuropathic pain and that global knockout of Tiam1 in mice prevented the development of neuropathic pain. Global knockout causes no other apparent abnormalities in the mice.
The UAB and Baylor researchers found that Tiam1 expression in the spinal dorsal horn neurons—but not in the dorsal root ganglion neurons or excitatory forebrain neurons—was essential for the development of neuropathic pain. Furthermore, they found that neuropathic pain development depended on Tiam1 expression in excitatory neurons—not in inhibitory neurons.
After showing where Tiam1 acts in neuropathic pain, Li, Tolias and colleagues showed what Tiam1 does. Tiam1 is known to modulate the activity of other proteins that help build or unbuild the cytoskeletons of cells, and the building of cytoskeleton actin filaments is part of dendritic spine creation. The researchers found that Tiam1 is necessary during the development of neuropathic pain to increase the density of dendritic spines on wide dynamic range neurons from the spinal dorsal horn and to increase synaptic NMDA receptor activity of spinal dorsal horn neurons.
Tiam1 functions to activate the small GTPase Rac1 enzyme that promotes actin polymerization. The researchers showed that the development of Tiam1-mediated neuropathic pain was dependent on Tiam1-Rac1 signaling. They then used a small molecule inhibitor to block Rac1 activation at three different time points—right after peripheral nerve injury, four days after nerve injury when neuropathic pain hypersensitivity gradually develops, or three weeks after nerve injury when chronic neuropathic pain is fully established. They found that neuropathic pain was prevented or reversed at each time point. Thus, Tiam1-Rac1 signaling is essential for the initiation, transition and maintenance of neuropathic pain.
Since Tiam1 appeared to be a promising therapeutic target for treating neuropathic pain, Li and Tolias also tested whether they could reduce neuropathic pain by injecting antisense oligonucleotides, or ASOs—short, synthetic, single-stranded oligodeoxynucleotides designed to alter Tiam1 expression by modulating its mRNA processing or degradation—into the cerebrospinal fluid of the spine.
In a rat model, they found that injecting an ASO against Tiam1 decreased Tiam1 protein levels in the spinal dorsal horn by 50 percent and significantly reduced neuropathic pain hypersensitivity one week after injection, a reduction that lasted another two weeks.
Therefore, Tiam1 is an essential player in the pathogenesis of neuropathic pain that coordinates actin cytoskeletal dynamics, dendritic spine morphogenesis and synaptic receptor function in spinal dorsal horn excitatory neurons in response to nerve damage, Li and Tolias say.
More information: Lingyong Li et al, Tiam1 coordinates synaptic structural and functional plasticity underpinning the pathophysiology of neuropathic pain, Neuron (2023). DOI: 10.1016/j.neuron.2023.04.010