This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
FDA approves subcutaneous Vyvgart Hytrulo for generalized myasthenia gravis
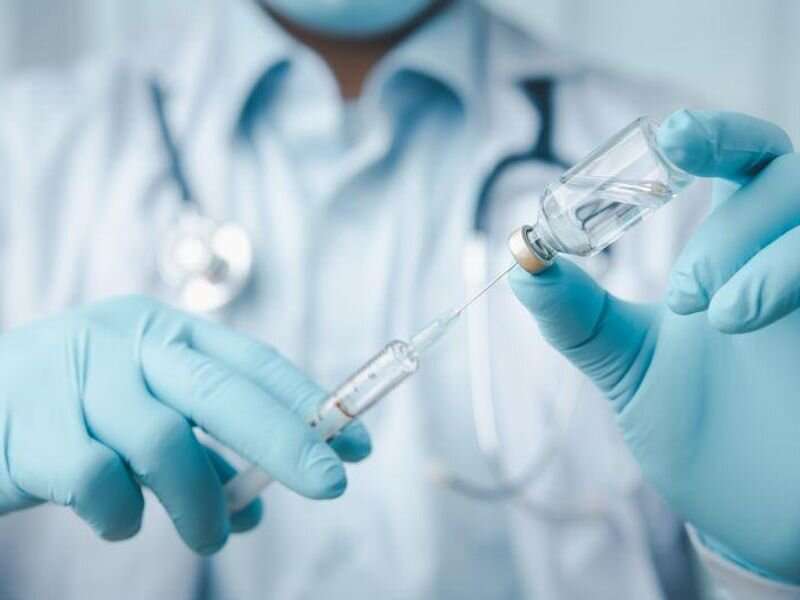
The U.S. Food and Drug Administration has approved Vyvgart Hytrulo (efgartigimod alfa and hyaluronidase-qvfc) subcutaneous injections for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.
Vyvgart Hytrulo is a combination of efgartigimod alfa, a human immunoglobulin (Ig)G1 antibody fragment, and recombinant human hyaluronidase PH20 (rHuPH20). The health care professional-administered subcutaneous injection (1,008 mg fixed dose) is given once a week for four weeks.
The approval was based upon the phase 3 ADAPT-SC study in which subcutaneous Vyvgart achieved reduction in anti-AChR antibody levels comparable to intravenous Vyvgart in adult gMG patients. From baseline, mean total IgG dropped 66.4 percent at day 29. The manufacturer of Vyvgart Hytrulo, argenx, says the treatment should be available to U.S. patients in July 2023.
"With our broad gMG offering of both a first-in-class infusion and subcutaneous injection, we continue to offer an individualized treatment approach and possibility of staying symptom free, while providing patients options of how and where they want to seek treatment," Luc Truyen, M.D., Ph.D., the chief medical officer at argenx, said in a statement.
Approval of Vyvgart Hytrulo was granted to argenx.
More information: More Information
Copyright © 2023 HealthDay. All rights reserved.