This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New images capture unseen details of the synapse
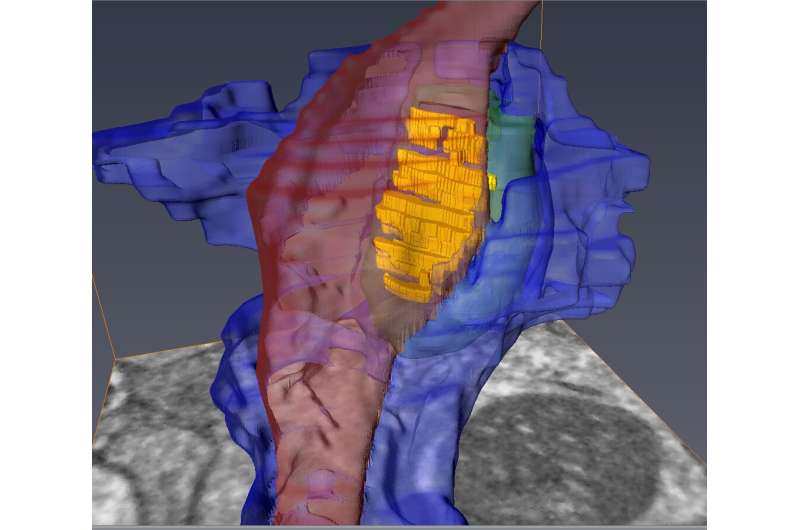
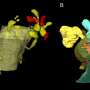
Scientists have created one of the most detailed 3D images of the synapse, the important juncture where neurons communicate with each other through an exchange of chemical signals. These nanometer-scale models will help scientists better understand and study neurodegenerative diseases such as Huntington's disease and schizophrenia.
The new study appears in the journal PNAS and was authored by a team led by Steve Goldman, MD, Ph.D., co-director of the Center for Translational Neuromedicine at the University of Rochester and the University of Copenhagen. The findings represent a significant technical achievement that allows researchers to study the different cells that converge at individual synapses at a level of detail not previously achievable.
"It is one thing to understand the structure of the synapse from the literature, but it is another to see the precise geometry of interactions between individual cells with your own eyes," said Abdellatif Benraiss, Ph.D., a research associate professor in the Center for Translational Neuromedicine and co-author of the study. "The ability to measure these extremely small environments is a young field, and holds the potential to advance our understanding of a number of neurodegenerative and neuropsychiatric diseases in which synaptic function is disturbed."
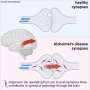
The researchers used the new technique to compare the brains of healthy mice to mice carrying the mutant gene that causes Huntington's disease. Prior research in Goldman's lab has shown that dysfunctional astrocytes play a key role in the disease. Astrocytes are members of a family of support cells in the brain called glia, and help maintain the proper chemical environment at the synapse.
The researchers focused on synapses that involve medium spiny motor neurons; the progressive loss of these cells is a hallmark of Huntington's disease. The researchers first had to identify synapses hidden within the tangle of the three different cells that converge at the site: the pre-synaptic axon from a distant neuron; its target, the post-synaptic medium spiny motor neuron; and the fiber processes of a neighboring astrocyte.
To do so, the investigators employed viruses to assign separate fluorescent tags to the axons, motor neurons, and astrocytes. They then removed the brains, imaged the areas of interest by multiphoton microscopy, and used a technique called infrared branding that employs lasers to create reference points in the brain tissue, which allowed the researchers to later relocate the cells of interest.
The team then examined the brain tissue using a serial block-face scanning electron microscope located at the University of Copenhagen, a research tool created to study the smallest structures of the brain. The device uses a diamond knife to serially remove and image ultrathin slices of brain tissue, creating 3D, nanometer scale models of the labeled cells and their interactions at the synapse.
"The models reveal the geometry and structural relationships between astrocytes and their partnered synapses, which is important because these cells must interact in a specific manner at the synapse," said Carlos Benitez Villanueva, Ph.D., senior associate in Center for Translational Neuromedicine and first author of the study. "This approach gives us the ability to measure and describe the geometry of the synaptic environment, and to do so as a function of glial disease."
In the brains of healthy mice, the team observed that astrocytic processes engaged with and completely enveloped the space around the disk-shaped synapse, creating a tight bond. In contrast, the astrocytes in Huntington's mice were not as effective in investing or sequestering the synapse, leaving large gaps. This structural flaw allows potassium and glutamate—chemicals that regulate communication between cells—to leak from the synapse, potentially disrupting normal cell-cell communication.
Astrocyte dysfunction been linked with other conditions, including schizophrenia, amyotrophic lateral sclerosis, and frontotemporal dementias. The researchers believe this technique could greatly improve our understanding of the precise structural basis for those diseases. In particular, they point out that this technique might be used to evaluate the effectiveness of cell replacement strategies, which replace sick glial cells with healthy ones, for treating these diseases.
Additional co-authors include Hans Stephensen and Jon Sporring with the University of Copenhagen, and Rajmund Mokso with Lund University in Sweden.
More information: Carlos Benitez Villanueva et al, Astrocytic engagement of the corticostriatal synaptic cleft is disrupted in a mouse model of Huntington's disease, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2210719120