This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study shows progress with multi-valent mRNA vaccines against monkeypox for enhanced protection
A team of researchers from the Chinese Academy of Sciences, the Chinese Academy of Agricultural Sciences, and Beijing Institute of Pharmacology and Toxicology have made significant progress in developing multi-valent mRNA vaccines against monkeypox virus (MPV), the agent that can cause smallpox-like disease in humans.
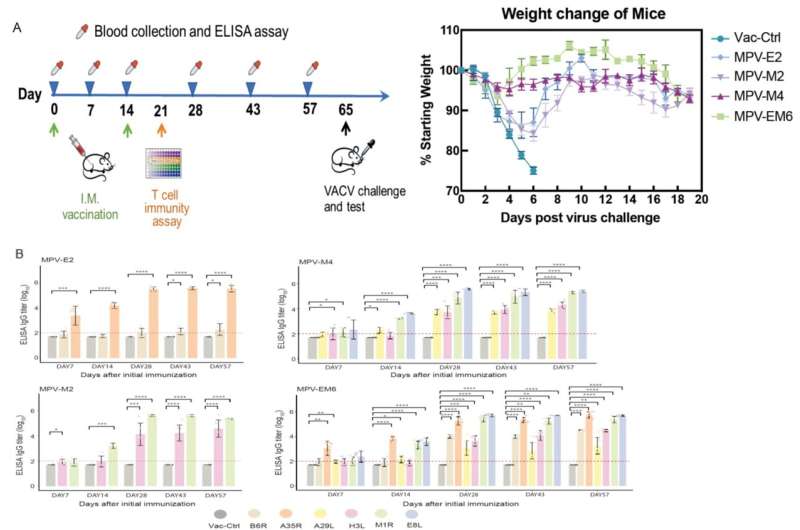
Their study showed the multi-valent mRNA vaccines with different combinations of monkeypox enveloped viron (EV) or mature viron (MV) surface antigens induced dynamic immune responses with a robust IgG response and correlating neutralizing activities. The vaccines protected a mouse model from a lethal dose vaccinia virus (VACV) challenge.
The study was published online in Science China Life Sciences. It provides insight into the protective mechanism of multi-valent mRNA vaccines and supports the development of effective and safe mRNA vaccines for enhanced protection against monkeypox virus outbreak.
Monkeypox, a virus that causes a disease similar to smallpox in humans, was first identified in African non-human primates in the 1950s. In recent years, the incidence of human-to-human transmission has increased, resulting in an expanding epidemic of monkeypox virus infection that spread to many countries around the world. Monkeypox was declared a global health emergency by the World Health Organization in July 2022.
The researchers first focused on the viral protein targets which are the key for vaccine development. They chose six MPV surface proteins, two from EV (A35R and B6R) and four from MV (A29L, E8L, H3L, and M1R), based on the studies of other orthopoxvirus and assessment of the immunogenicity potential of MPV surface proteins using an in silico computation approach.
The multi-valent mRNA vaccines with EV-only, MV-only, or EV+MV combined antigens were administered in Balb/c mice to assess their safety and immunogenicity potentials. A dynamic immune response was observed as soon as seven days after initial immunization, while the antibody responses for all the EV- and MV-surface antigens were significantly boosted with the second immunization.
Researchers also found that the EV+MV combined immunogens contributed to a more robust total IgG response and correlating neutralizing activity against VACV, indicating the additive potential of each immunogen in generating immune response and nullifying VACV infection. Furthermore, the mRNA vaccines were shown to elicit an antigen-specific CD4+ T cell response that is biased towards Th1.
"Amazingly, in a mouse model challenged with a lethal dose VACV, all the mice immunized with the mRNA vaccines (with various combinations of EV and MV surface antigens) survived VACV infection." explained Dr. Pei Hao, leading researcher of the collaborative study and senior author of the published paper.
"Particularly the strongest protection was seen with the EV and MV surface antigens-combined vaccine (MPV-EM6), which shows an excellent correlation with the total IgG response and corresponding neutralizing activity (against VACV). It is a good indication that our multi-valency approach is paying off."
The swift responsible time and flexibility of mRNA vaccines provide an un-paralleled platform for rapid development and deployment of vaccines against any threat of pathogenic outbreaks. This study highlights its significance in combating pathogenic outbreaks and focused on the development of multi-valent mRNA vaccines targeting the monkeypox virus.
These findings offer valuable insights into the protective mechanisms of multi-valent mRNA vaccines and lay the ground work for future development of effective and safe mRNA vaccines for enhanced protection against monkeypox virus outbreak.
More information: Niubing Zhang et al, Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity, Science China Life Sciences (2023). DOI: 10.1007/s11427-023-2378-x