This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Neuroscientists identify molecule that acts as a 'hub' when it's time to communicate
Neuroscientists have uncovered a new function for a molecule that is found to be essential for brain communication, which could potentially improve brain function in individuals with autism and neurodevelopmental disorders.
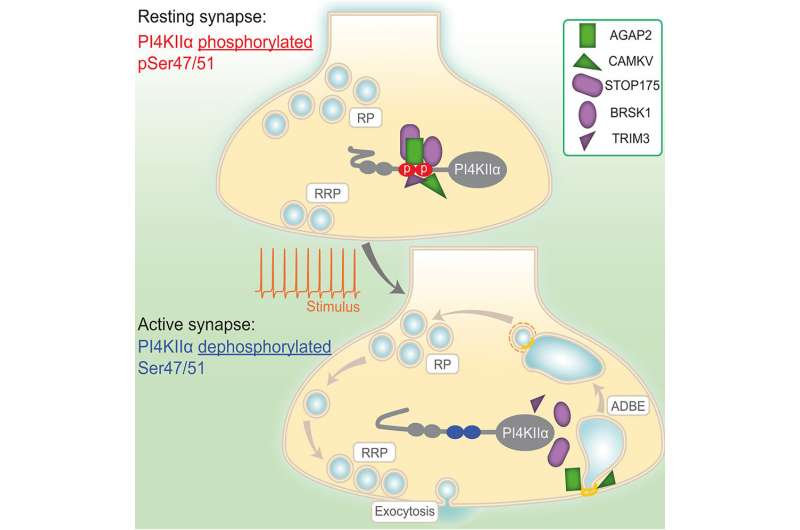
Researchers at the Simons Initiative for the Developing Brain found that brain activity modifies the function of a specific protein called phosphatidylinositol-4 kinase (PI4-kinase). In resting brain cells, this protein acts like a magnet to attract other molecules, and then during activity, it disperses these molecules to do their jobs. This event sustains a process called neurotransmitter release which is the primary mechanism of brain communication. Specifically, PI4-kinase sustains neurotransmitter release during periods of high brain activity by regulating a process called 'bulk endocytosis."
The study, published in Cell Reports, describes the molecular mechanism behind this magnetic ability, namely the addition of a phosphate group to PI4-kinase by an enzyme called GSK3 (glycogen synthase kinase 3).
"We knew from our previous work that PI4-kinase was a protein that gets modified by GSK3. Since we knew that GSK3 was essential for bulk endocytosis, we thought that its modification of PI4-kinase may have an important role in this process too," said Professor Mike Cousin, Group Leader at the Simons Initiative for the Developing Brain at The University of Edinburgh and co-corresponding author on the paper.
In this study, the Cousin lab carried out experiments to measure bulk endocytosis activity. They added fluorescent molecules (that were designed to specifically report bulk endocytosis) to brain cells grown on glass coverslips during high activity. These brain cells were manipulated to remove the PI4-kinase and then reintroduce different forms of PI4-kinase with specific functions inhibited.
The team found that the addition or removal of a phosphate group from PI4-kinase was the only manipulation which altered bulk endocytosis. This led to the model where in a resting neuron, PI4-kinase has a phosphate group and recruits molecules important for bulk endocytosis. However, during high brain activity, PI4-kinase loses its phosphate group, which results in liberation of those molecules to perform their jobs.
The next steps for the researchers are to address two of the molecules that are liberated on dephosphorylation of PI4 kinase—AGAP2 and CAMKV, since both were shown in the paper to be essential for bulk endocytosis. AGAP2 is a gene disrupted in autism, suggesting another link between bulk endocytosis and this neurodevelopmental condition. There is a student who is currently researching this very link.
More information: Eva-Maria Blumrich et al, Phosphatidylinositol 4-kinase IIα is a glycogen synthase kinase 3-regulated interaction hub for activity-dependent bulk endocytosis, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112633