This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New research reveals the start of Huntington's disease
Devastating neurodegenerative diseases like Huntington's, Alzheimer's, and Parkinson's are all associated with protein deposits in the brain, known as amyloid. Despite extensive research investment into the cause and toxicity of amyloids, deciphering the first step in formation along with effective therapies has remained elusive.
For the first time, scientists at the Stowers Institute for Medical Research have uncovered the structure of the first step in amyloid formation, called the nucleus, for Huntington's disease. The study published in eLife from the lab of Associate Investigator Randal Halfmann, Ph.D., proposes a new, radical method for treating not only Huntington's but potentially dozens of other amyloid-associated diseases—preventing the initial, rate-limiting step from occurring.
"This is the first time anyone has experimentally determined the structure of an amyloid nucleus even though most major neurodegenerative diseases are associated with amyloids," said Halfmann. "One of the big mysteries of Huntington's, Alzheimer's, and ALS is why disease coincides with amyloid, yet the amyloids themselves are not the main culprits."
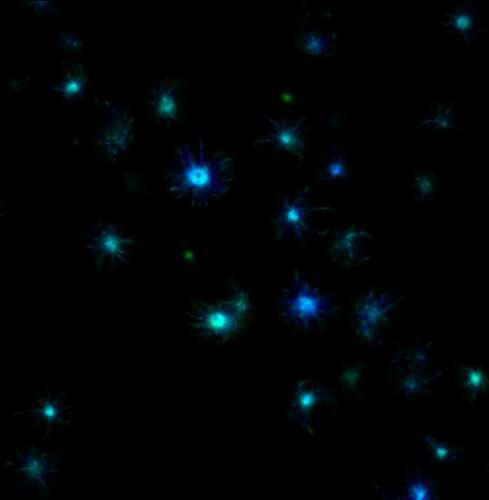
Co-first authors Tej Kandola, Ph.D., and Shriram Venkatesan, Ph.D., uniquely identified the structure of the amyloid nucleus for huntingtin, the protein responsible for Huntington's disease, discovering that the nucleus forms within a single protein molecule.
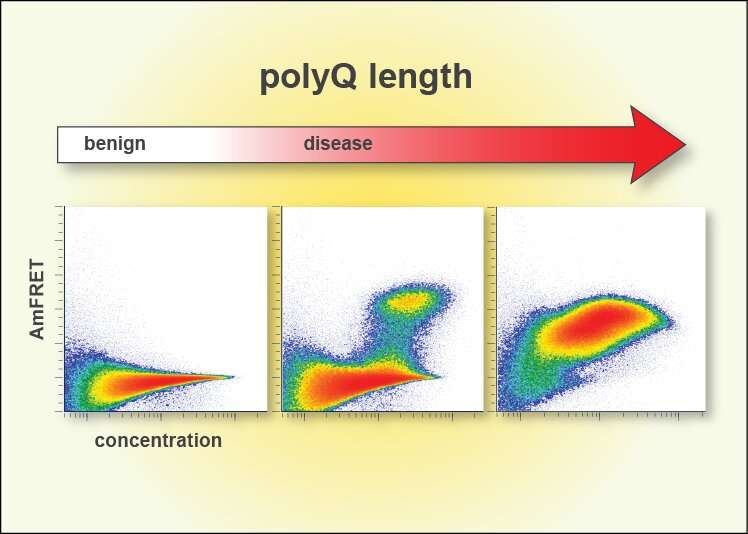
Proteins are the cell's factory workers constructed from unique sequences of 20 amino acids, their building blocks. Some proteins have repeats of one of these amino acids—glutamine (abbreviated as Q). Huntington's and eight other diseases, collectively called "PolyQ diseases," occur when certain proteins have a repeat that is too long. Somehow, this causes the proteins to fold into a specific structure that starts a chain reaction that kills the cell.
"For three decades, we've known that Huntington's and related fatal diseases occur when proteins contain more than around 36 Qs in a row, causing them to form chains of proteins in the brain, but we didn't know why," said Halfmann. "We've now figured out what the first link in the chain looks like, and, in doing so, have discovered a new way to stop it."
"I am, frankly, astonished that such an intuitive physical model of nucleation emerged despite the intrinsic complexity of the cellular environment," said Professor Jeremy Schmit, Ph.D., from Kansas State University.
"I am truly excited by the intuition and the testable hypotheses that this work inspires."
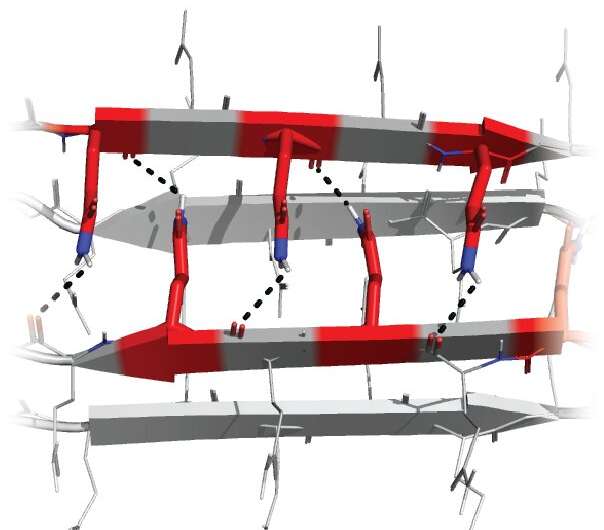
A paradigm shift and potential therapeutic method
These new findings are potentially a paradigm shift for how we view amyloids. The results from this research suggest that it is the early committed steps of amyloid formation, right after the nucleus forms, that cause neuronal cell death.
Along with uncovering the key structure that begins polyQ amyloid formation, researchers found that it only formed in isolated molecules of the protein. Clumping the proteins together in cells stopped amyloids forming altogether. This is a novel therapeutic avenue the team plans to explore further in mice and brain organoids.
A new technique
A technique recently developed by the Halfmann Lab, Distributed Amphifluoric Förster Resonance Energy Transfer (DAmFRET), shows how a protein self-assembles in single cells. This method turned out to be crucial for observing the rate-limiting amyloid-forming nucleation event.
"A key innovation was to minimize the volume of the reaction to such an extent that we can witness its stochasticity, or randomness, and then we tweak the sequence to figure out what is governing that," said Halfmann.
Designing and testing specific patterns of Qs enabled the team to deduce the minimum structure that could form amyloid—a bundle of four strands each with three Qs in specific locations. This tiny crystal inside a single molecule of the protein is the first step in a chain reaction that results in disease.
"Prior work in test tubes supports a monomeric nucleus, but this model has been controversial," said Halfmann. "We now have strong evidence that 36 Qs is the critical number for nucleation to happen in single protein molecules, and moreover, that this is how it happens inside living cells."
In essence, this work provides a molecular model to investigate the structure of any amyloid nucleus. Additionally, the correlation between aging and amyloids suggests that this method may ultimately uncover molecular mechanisms that cause aging. The preemptive approach to eliminate or at the very least to delay nucleation provides hope for people with pathologic PolyQ proteins.
"The emerging paradigm is that everything follows from a single event, a spontaneous change in protein shape," said Halfmann. "That event ignites the chain reaction for amyloids that kill cells and may provide critical insight into how amyloids cause disease."
More information: Tej Kandola et al, Pathologic polyglutamine aggregation begins with a self-poisoning polymer crystal, eLife (2023). DOI: 10.7554/eLife.86939.1