This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Fresh insights into how glucose drives tuberculosis vaccine responses offer hope for improved efficacy
BCG is a live bacterial vaccine, of limited effectiveness for tuberculosis, but it's the only one we've got. However, scientists in the TB Immunology group at Trinity College Dublin and St. James's Hospital have provided fresh insights into the behavior of a crucial cell in vaccine mechanisms, which may offer a fresh target for scientists seeking to improve vaccine efficacy.
The Trinity and St. James's team have just published their findings, focused on the behavior of CD1c+ myeloid dendritic cells, in the journal Frontiers in Cellular and Infection Microbiology.
Their work outlines an improved understanding of how the body uses glucose to drive immunity after BCG vaccination.
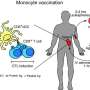
BCG is the most commonly used vaccine in the world. After vaccination with BCG, myeloid dendritic cells in the skin engulf the bacteria and transport them to lymph nodes where they interact with T cells to initiate protective immunity.
Dendritic cells are sparsely distributed throughout the body, however, which creates a challenge for researchers who wish to study them. Using blood donated by volunteer patients at St. James's, Dr. Denise Triglia, a Research Fellow who carried out the work in the Trinity Translational Medicine Institute (TTMI) and first author on the paper, devised a method to purify enough CD1c+ myeloid dendritic cells to carry out functional studies.
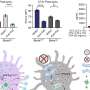
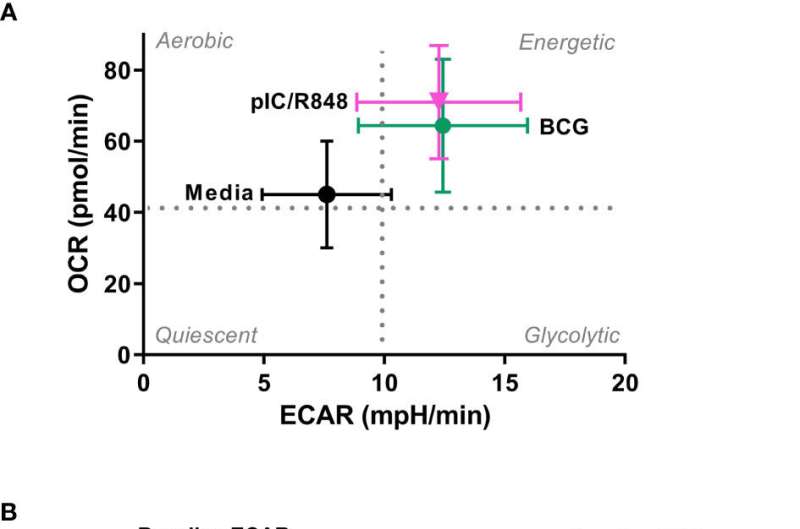
And in a series of cellular experiments, she and the wider team have implicated a key role for glucose metabolism in how these cells deal with infection by BCG.
After carefully studying how the dendritic cell responds, they found that glucose metabolism improves the ability of this immune cell to drive certain beneficial vaccine responses. At the same time, they noticed that glucose metabolism was not required for the migration of infected dendritic cells in response to the chemical messenger CCL19 and may, in fact, block their migration.
Dr. Mary O'Sullivan, who led the research team, said, "Dendritic cells change the way they metabolize glucose after encountering a vaccine or pathogen, which, in turn, influences the outcome of the subsequent immune response. This is the first study examining the role of metabolism in human CD1c+ myeloid dendritic cells exposed to BCG and our results highlight the complex relationship between cellular metabolism and dendritic cell function. Understanding how BCG affects dendritic cell metabolism could help us to design new vaccines that stimulate a better immune response."
Global health has benefited from the research-driven rapid production of an effective COVID-19 vaccine and it is now evident that TB vaccine development, which is lagging behind, requires new ideas to generate a more effective vaccine than BCG.
Professor Joseph Keane, who leads the TB Immunology group in TTMI, said, "The metabolic manipulation of the dendritic cell now becomes an option to try and improve vaccine efficacy for this disease, which is overtaking COVID-19 as the single biggest global infectious killer."
More information: Denise Triglia et al, Glucose metabolism and its role in the maturation and migration of human CD1c+ dendritic cells following exposure to BCG, Frontiers in Cellular and Infection Microbiology (2023). DOI: 10.3389/fcimb.2023.1113744