This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Hypothermic neuroprotection by targeted cold autologous blood transfusion in a non-human primate stroke model
Therapeutic hypothermia is a well-established therapy with clear neuroprotective effects. It has been widely used in cases of neonatal hypoxic encephalopathy and cardiac arrest. Researchers have been attempting to apply therapeutic hypothermia to the treatment of acute ischemic stroke for decades. However, many hypothermia induction methods that have proven effective in rodent transient cerebral ischemia models have encountered challenges in clinical translation.
The reason may lie in the differences between rodents and humans in brain volume and metabolism, as well as a mismatch between hypothermia induction methods performed in clinical and preclinical trials. Therefore, how to induce hypothermia safely and efficiently in an appropriate model is the key to the clinical translation of therapeutic hypothermia.
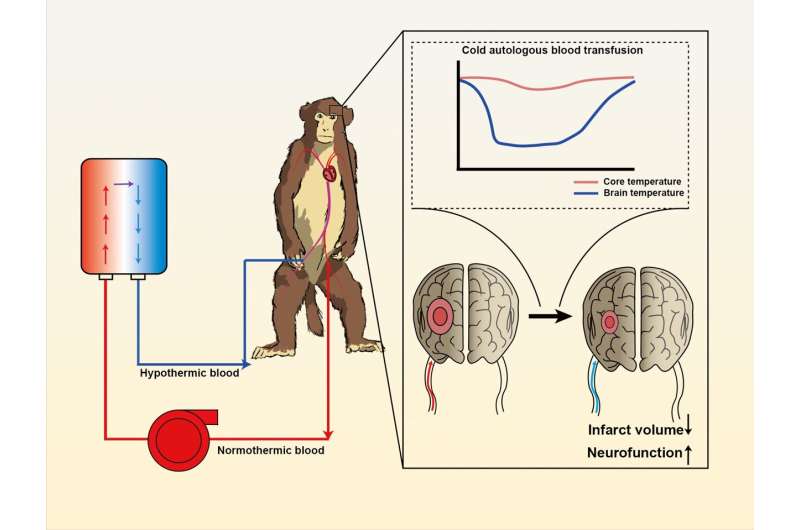
In a study published in Science Bulletin, a rhesus monkey model of transient ischemia-reperfusion was established to simulate the pathogenesis and treatment of ischemic stroke by placing/withdrawing endovascular coils. Researchers extracted arterial blood from the left femoral artery of the monkeys, passed it through a heat exchange unit they had developed, and then transfused it directly into the recanalized cerebral artery via a microcatheter placed previously in the right femoral artery.
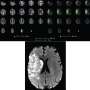
To evaluate the neuroprotective effect of cold autologous blood, the researchers used magnetic resonance imaging (MRI) to evaluate the infarct volume of the animals. Targeted cold autologous blood transfusion effectively reduced infarct volume and infarct core expansion in the acute phase of ischemic stroke, as shown on T2 sequence and diffusion weighted imaging (DWI).
In addition, diffusion tensor imaging (DTI) showed that hypothermia preserved white matter integrity. Consistent with reduced infarct volume, cold autologous blood also improved neurological function.
This method could be used to reduce brain temperature rapidly (down to 34°C within 5 minutes) while maintaining relatively higher core body temperature during a two-hour procedure. More importantly, no adverse effects of systemic hypothermia—such as shivering and infection—were observed, indicating the safety of this method.
More information: Jian Chen et al, Hypothermic neuroprotection by targeted cold autologous blood transfusion in a non-human primate stroke model, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.06.017