July 6, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Platinum chemotherapeutics: A sonoactivated platinum anticancer prodrug
While photoactivated chemotherapy has received significant attention, the capacity to eradicate a deep tumor by using an external source that provides high penetration depth of tissue remains challenging. In a new report now published on Science Advances, Gongyuan Liu and a team of scientists in chemistry, biomedical engineering and bioengineering at the City University of Hong Kong in China presented cyaninplatin as a Platinum (IV) anticancer prodrug.
The drug molecule can be activated by ultrasound by employing a precise mechanism-of-action for precision oncotherapy. After sonoactivation, the cyaninplatin drug showed strengthened mitochondrial DNA damage as a marker of cell killing efficiency.
The prodrug overcame drug resistance as a result of combinatorial therapy mechanisms, which included the release of platinum chemotherapeutics, the breakdown of intracellular reductants, and a large release of reactive oxygen species.
This combined approach is known as sono-sensitized chemotherapy. The process was guided by high-resolution ultrasound, optical and photoacoustic imaging methods for tumor theranostic strategies in vivo with superior efficiency and biosafety. The work highlighted the significance of ultrasound to specifically activate the platinum anticancer drugs, eradicate deep tumor lesions and expand theranostics of platinum coordinate complexes.
Therapeutic courses
Liu and colleagues used energy-conversion chemistry to activate the prodrugs by using exogeneous stimuli to realize therapeutic courses. The molecular prodrugs augmented therapeutic behavior of the original drug moieties to accomplish personalized, location-specific treatment with reduced side-effects.
Photoactivatable platinum (IV) prodrugs are therapeutically advantageous since they originate from platinum anticancer prodrugs for high stability and easy functionalization.
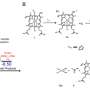
![Stability and focused ultrasound (FUS)–mediated reduction of cyaninplatin. (A) Chemical structure of cyaninplatin. (B) Stability of cyaninplatin in complete RPMI 1640 culture medium containing 10% fetal bovine serum (FBS) at 37°C. (C) Stability of cyaninplatin in the lysate of A2780 cells at 37°C. (D) Reduction of cyaninplatin under sono-activation (FUS: 1.75 MHz, 4 W, 0 to 60 min) in phosphate-buffered saline (PBS) containing 5 mM ascorbate at 37°C with (E) corresponding reduction profile of cyaninplatin with or without FUS. (F) Liquid chromatography (LC) trace of reduction products of sono-activated (4 W, 45 min, in PBS buffer, pH 7.4, with 5 mM ascorbate, ultraviolet-visible (UV-vis) detector at 700 nm) cyaninplatin [insert: LC trace and mass spectrometry (MS) results of released carboplatin], with corresponding HR-MS analysis results of (G) peak 1 and (H) peak 2. (I) Calf thymus DNA (ct-DNA) binding of carboplatin or cyaninplatin with or without FUS activation (4 W, 60 min, in PBS buffer, pH 7.4, with 5 mM ascorbate). t test, N.S. not significant, mean ± SD, n = 3. a.u., arbitrary units; m/z, mass/charge ratio. Credit: Science Advances, doi: 10.1126/sciadv.adg5964 Platinum chemotherapeutics: A sonoactivatable platinum anticancer prodrug](https://scx1.b-cdn.net/csz/news/800a/2023/platinum-chemotherapeu-1.jpg)
Platinum scaffolds can be combined with phototherapy to generate radicals or reactive oxygen species for therapeutic applications. While photoactivatable platinum drugs have been successful, their light penetration depth is too shallow to treat deep tumors. The presence of strong optical scattering can also prevent precision medicine treatment of deep tissue.
In this work, Liu and colleagues presented an ultrasound-activated platinum (IV) prodrug for on-demand release of platinum chemotherapeutics using a sono-sensitized electron transfer process. The method also included a focused ultrasound system.
The prodrug additionally functioned as a multi-imaging contrast agent to accomplish high-resolution ultrasound imaging, near-infrared optical imaging and photoacoustic computed tomography prior to therapeutic intervention. The process of cyaninplatin activation demonstrated outstanding cancer efficacy in a mouse model to facilitate promising sono-sensitized chemotherapy for non-invasive treatment of deep tumors.
Synthesizing and characterizing the platinum prodrug
The research team obtained a representative heptamethine cyanine sonosensitizer known as IR780 to rationally design an ultrasound-responsive theranostic platinum coordination compound. This compound efficiently entered cells through organic anion-transporting polypeptides to accumulate in the cellular mitochondria. The scientists used a carboplatin-based platinum scaffold for stability and modified IR780 to create a ligand.
They named the resulting platinum complex cyaninplatin and characterized the purified complex by using nuclear magnetic resonance, ultraviolet, visible and fluorescence spectroscopies, as well as liquid chromatography-high resolution mass spectroscopy. This compound—cyaninplatin can also serve as a contrast agent for optical and photoacoustic imaging to deliver a theranostic approach.
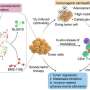
![Cell-based in vitro experiments. (A) Cellular accumulation of cyaninplatin (12.5 μM) and carboplatin (12.5 μM). (B) Uptake and infiltration of cyaninplatin in 4T1 tumor spheroids (25 μM, 30 min). (C) Subcellular distribution of cyaninplatin (12.5 μM, 30 min). (D) Pt contents in mitochondrial parts of cells (drug feeding: 12.5 μM, 30 min). (E) Relative cell viability under different treatment conditions: 1. vehicle control [0.5% dimethylformamide (DMF)], 2. focused ultrasound (FUS)(3.5 W, 15 min), 3. cisplatin (12.5 μM), 4. carboplatin (12.5 μM), 5. carboplatin + FUS, 6. ligand 1 + carboplatin, 7. ligand 1 + carboplatin + FUS, 8. cyaninplatin (12.5 μM), 9. cyaninplatin + FUS. (F) Relative glutathione (GSH) level with different treatment (drug feeding: 25 μM, 30 min; FUS: 3.5 W, 10 min). (G) Oxidative depletion of nicotinamide adenine dinucleotide (NADH) with different treatment (drug feeding: 25 μM, 30 min; FUS: 3.5 W, 10 min). (H) Pt-binding level of mitochondrial DNA (mtDNA) of cells treated with carboplatin + ligand 1 or cyaninplatin (12.5 μM, 30 min) with FUS (3.5 W, 15 min). (I) Quantitative result of JC-1 staining (drug feeding: 25 μM, 30 min; FUS: 3.5 W, 10 min). (J) DCFH-DA staining for reactive oxygen species (ROS) levels upon various treatments (cyaninplatin at 25 μM, 30 min; FUS: 3.5 W, 10 min). (K) Calcein-acetoxymethyl (AM)/PI double staining of cells received different treatments (drug feeding: 25 μM, 30 min; FUS: 3.5 W, 10 min). 4T1 cells were used in all these assays. Mean ± SD, t test, **P < 0.01, ***P < 0.001, n = 3. Credit: Science Advances, doi: 10.1126/sciadv.adg5964 Platinum chemotherapeutics: A sonoactivatable platinum anticancer prodrug](https://scx1.b-cdn.net/csz/news/800a/2023/platinum-chemotherapeu-2.jpg)
Additional experiments
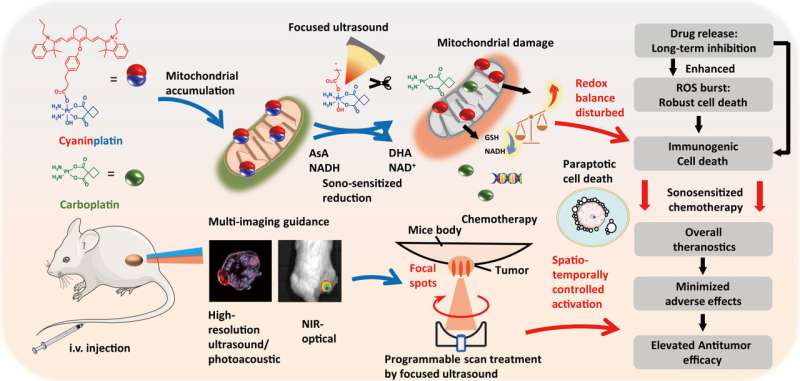
Liu and colleagues conducted a series of experiments to explore the stability and sonoactivation of the drug, monitored its capacity for cellular uptake and subcellular distribution, alongside cytotoxicity against different cancer cell lines. Using HeLa cells; an immortalized cancer cell line, the scientists studied cell apoptosis on cell death mode induced by sonoactivated cyaninplatin.
Since the specific mode of cancer cell destruction known as paraptosis is related to mitochondrial dysfunction and oxidative stress in the endoplasmic reticulum through photodynamic therapy, the scientists used MitoTracker dye and an endoplasmic-reticulum tracker to stain the cyaninplatin-treated cells. Using imaging methods, they confirmed cell swelling and severe mitochondrial dysfunction, to show a benefit of anticancer efficiency suited for in vivo studies.
In vivo anticancer activity
The researchers next established a synergetic model based on the 4T1 murine mammary carcinoma cell line to resemble stage IV breast cancer in an immunocompetent mouse model. A 12-hour post-injection window provided an optimal therapeutic timeframe for sonoactivation. The scientists treated the tumors with focused ultrasound via programmable scanning to move the focus of ultrasonication to cover the entire region of interest.
-
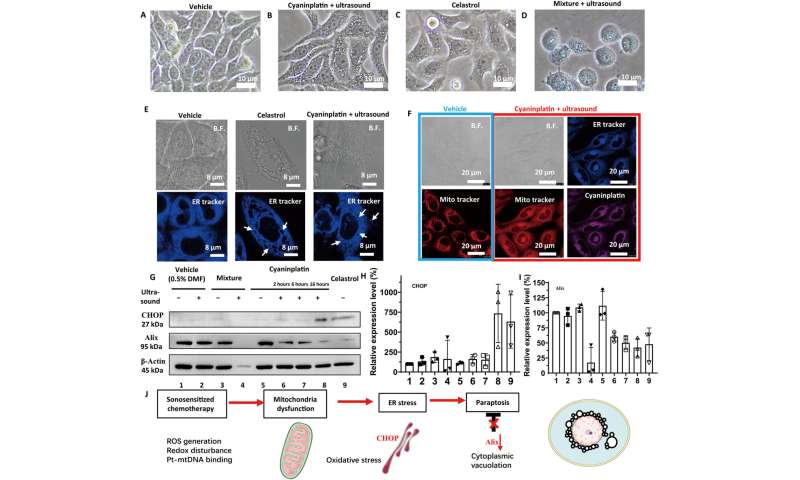
Exploration of cancer cell death mode. Bright-field (B.F.) images of HeLa cells under various treatments. (A) Vehicle control (0.5% DMF), (B) cyaninplatin (10 μM, 30 min) + focused ultrasound (FUS) (3 MHz, 3.5 W, 15 min), (C) celastrol (2 μM, 6 hours), and (D) carboplatin + ligand 1 (35 μM, 30 min) + FUS. 5. Carboplatin + FUS, 6. ligand 1 + carboplatin, 7. ligand 1 + carboplatin + FUS. (E) Confocal laser scanning microscopy (CLSM) images of cells with cytoplasmic vacuolation and (F) depolarization of mitochondria. (G) Western blot and (H and I) quantitative results of HeLa cells with different treatment conditions. Drug feeding: mixture of carboplatin + ligand 1 (35 μM, 30 min); cyaninplatin (10 μM, 30 min); celastrol (2 μM, 6 h). FUS: (3 MHz, 3.5 W, 15 min). (J) Proposed mechanism for sono-activated cyaninplatin to induce paraptosis. Mean ± SD, n = 3. Credit: Science Advances, doi: 10.1126/sciadv.adg5964 -
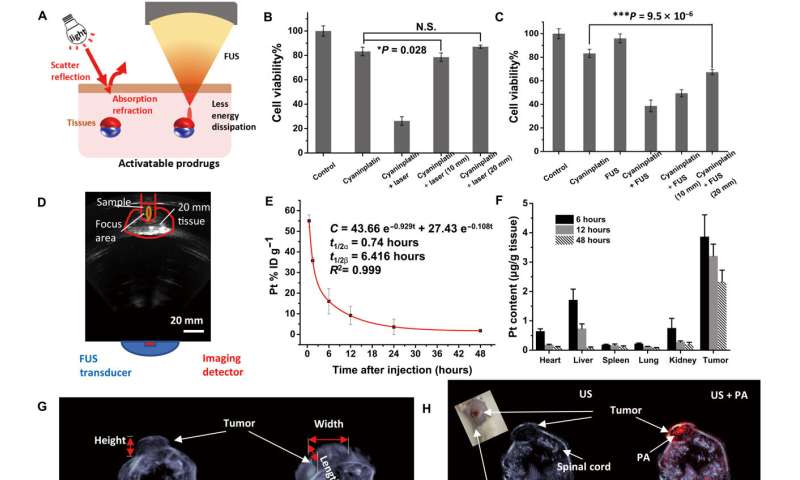
Circulation, biodistribution, and multimodal imaging. (A) Schematic illustration of tissue penetration process of light and focused ultrasound (FUS). (B) Tissue penetration test of near-infrared (NIR) laser-activated cyaninplatin against chicken breast tissues (HeLa cells, drug feeding: 12.5 μM, 30 min; laser: 808 nm, 0.33 W cm−2, cell viability was tested at 24 h post-treatment). (C) Tissue penetration test of sono-sensitized chemotherapy (SSCT) efficacy against chicken breast tissues (drug feeding: 12.5 μM, 30 min; FUS: 3.5 W, 15 min, cell viability was tested at 24 hours after treatment) and (D) corresponding B-mode ultrasound imaging to monitor the treatment process. (E) Blood circulation for mice with intravenous injection of cyaninplatin. (F) Biodistribution of cyaninplatin at 6, 12, and 48 hours after injection of cyaninplatin. (G) Semi-3D reconstruction of tumor area by high-resolution ultrasound (US) imaging. (H) Photoacoustic (PA) computed tomography to verify the accumulation of cyaninplatin in the tumor at 9 hours after injection. (I) Quantitative result of photoacoustic signal in the tumor region after injection of cyaninplatin. (J) NIR optical imaging to verify tumor accumulation of cyaninplatin and (K) corresponding quantitative data. Mean ± SD, t test, *P < 0.05, ***P < 0.001, n = 3. Credit: Science Advances, doi: 10.1126/sciadv.adg5964
Liu and colleagues monitored the tumorigenic region and prevented hypothermia-related damage to adjacent tissues. Mice treated with sonoactivated cyaninplatin had predominantly inhibited tumor growth when compared to those without similar therapeutic intervention. The scientists further showed how sonoactivated cyaninplatin destroyed tumor tissue, while decreasing the level of tumor proliferation factor, to prevent tumor recurrence.
The outcomes emphasized successful T-cell infiltration in the tumor treated with sono-activated cyaninplatin, when compared to those treated with carboplatin and ligand 1 alone without sonoactivation.
The researchers also monitored tumor accumulation of cyaninplatin and the tissue penetration ability of focused ultrasound both in vivo and ex vivo. They confirmed the activation of cyaninplatin through ultrasound and confirmed the detection of reactive oxygen species by using singlet oxygen sensor green with great potential for clinical translation.
Outlook
In this way, Gongyuan Liu and colleagues developed cyaninplatin, a platinum (IV) prodrug activated and regulated by ultrasound. They achieved a molecular design architecture in a carrier-free manner for the drug to be internalized by cancer cells and accumulated in mitochondria. Once the pro-drug underwent irradiation with ultrasound during therapeutic intervention, it reduced to chemotherapeutic carboplatin via a sono-sensitized electron transfer process.
In its mechanism of action, the sono-activated cyaninplatin generated reactive oxygen species and depleted intracellular reductants to mediate mitochondrial damage and cancer cell killing efficiency. This study further expands existing understanding of the sonoactivation process of small molecules to broaden the scope of biomedical ultrasound and sono-activatable prodrugs as additional therapeutic options for cancer medicine.
More information: Gongyuan Liu et al, An ultrasound-activatable platinum prodrug for sono-sensitized chemotherapy, Science Advances (2023). DOI: 10.1126/sciadv.adg5964
Jin Geng et al, Switching on prodrugs using radiotherapy, Nature Chemistry (2021). DOI: 10.1038/s41557-021-00711-4
© 2023 Science X Network