July 31, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists engineer cooperation in cancer cells to activate apoptosis mechanisms
Scientists at Stanford University have found a way to induce cell death in cancer cells with a method that could be effective in around 50% of cancers. In a paper, "Rewiring cancer drivers to activate apoptosis," published in Nature, the team describes a new class of molecules called transcriptional/epigenetic CIPs (TCIPs) that can activate apoptosis with the help of cancer growth gene expressions within the cancer cells.
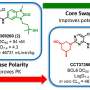
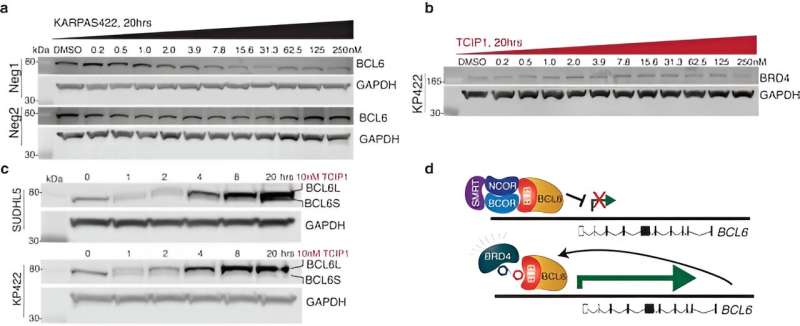
The researchers designed small molecules that bind specific transcriptional suppressors to transcription activators. The most potent molecule created, TCIP1, works by linking small molecules that bind BCL6 to those that bind transcriptional activators BRD4.
One of the components that makes cancer cells cancerous is that they ignore signals from surrounding healthy tissues to stop growing and to initiate apoptosis or cell death. The apoptosis pathways still exist but are actively blocked in certain types of cancer where the transcription factor B cell lymphoma 6 (BCL6) binds to the promoters of apoptosis genes and suppresses their expression through epigenetic mechanisms.
The researchers developed TCIPs by linking molecules that bind BCL6 with molecules that bind to the transcriptional activator BRD4. The normal activity of a transcriptional activator, like BRD4, in cancer cells is to promote rapid growth and proliferation of cells. When linked to BCL6, BRD4 will activate whatever BCL6 binds to, turning on any gene expression the cancer cell attempts to suppress with BCL6, including apoptosis genes.
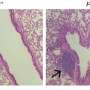
TCIP1 successfully killed large B cell lymphoma cell lines, including chemotherapy-resistant TP53-mutant lines and exhibited cell-specific and tissue-specific effects. The activation of apoptosis to cell death took place in just 72 hours.
BCL6 is critical to the lymphatic system, and mice engineered without the BCL6 gene die of complex inflammatory reactions. BRD4 is heavily involved in genome function and stability across many processes. Concerns regarding utilizing these important gene expressions and how they might affect off-target healthy tissues were addressed in the study.
TCIP1 acts in a context-specific manner requiring the expression of BRD4 and BCL6 to both be present in order to bind them and operate at a concentration that would occupy only a tiny fraction of the total BCL6 molecules.
In a mouse model, TCIPs were found to be non-toxic in cells lacking BCL6. Triple-negative breast cancer cell lines without BCL6 were not affected by the TCIP design.
TCIP1 induced dramatic transcriptomic changes in the spleen, notably with an upregulation of the FOXO3 gene, which mirrored activity in the targeted cancer cells. Despite the significant transcriptomic changes in the spleen, TCIP1 was well tolerated without adverse effects, with no significant differences in mouse body weight and no noticeable abnormalities such as inflammatory infiltrates or apoptotic cells.
TCIPs produce their effect by rewiring only a fraction of the cancer driver molecules per cell. With dosage concentrations used in the study, TCIP1 elevated BRD4 to a 1.5-fold increase at BCL6 sites and showed little interference in regular activity elsewhere while engaging in robust gene activation and cell killing within cancer cells. This suggests that the TCIPs could be developed into a therapeutic though more robust research is required to demonstrate safety.
According to the authors, the activation of genes by small-molecule TCIPs might have applications for many other areas of biology and medicine. For example, TCIPs could be designed for use in activating death pathways in senescent cells, activating the expression of therapeutic or haploinsufficient genes, activating the expression of neoantigens in human immunotherapy, or regulating gene expression in cells or organisms for synthetic biology applications.
While it is difficult to assess the therapeutic potential of a novel approach at this early stage of development, it is clearly innovative and opportunistic of mechanisms readily available and accessible across a wide range of pathologies.
More information: Sai Gourisankar et al, Rewiring cancer drivers to activate apoptosis, Nature (2023). DOI: 10.1038/s41586-023-06348-2
James D. Phelan et al, Double-headed molecule activates cell-death pathways in cancer cells, Nature (2023). DOI: 10.1038/d41586-023-02213-4
© 2023 Science X Network