July 24, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Spatial positioning of enzyme helps natural killer cells distinguish healthy cells from diseased ones marked for death
Questions abound when it comes to the molecular mechanisms deployed by the human immune system to thwart cancer and infection, and questions especially abound when it comes to the precise steps taken by the innate immune response, the first flurry of activity when invasive disease occurs.
Now, scientists have taken a deep dive into the activity of natural killer cells, key components of the innate immune system and a first responder on the scene when cancer cells or pathogens compromise healthy tissue. The innate immune system is not only the body's first line of defense, it is also present at birth. And because it always responds with the same relative force regardless of the abnormality, it is sometimes called the nonspecific immune system.
An international team of scientists, led by researchers at the Karolinska Institutet in Sweden, has found that the spatial distribution of an enzyme inside natural killer cells is critical to its functions. Indeed, findings by this far-flung team of scientists who were located in Sweden, Switzerland and Japan, suggest that the spatial positioning of the enzyme, which is called SHP-1, is critical to natural killers being able to distinguish cells that are compromised by disease from those that are healthy and unscathed by infection or malignancy.
"Natural killer cells recognize virally infected cells and tumor cells," writes Dr. Laurent Schmied and colleagues in the journal Science Signaling. "Natural killer cell function depends on balanced signaling from activating receptors, recognizing products from tumors or viruses, and inhibitory receptors, which recognize major histocompatibility complex class I—MHC-I—molecules."
Though natural killer cells may be part of the nonspecific immune system, they possess a high degree of specificity when it comes to recognizing and killing cells that are infected with viruses or compromised by cancer.
They do this, according to Schmied and collaborators, by forming contacts called synapses with compromised cells and releasing enzymes to kill these diseased targets. The process by which natural killers cells develop the ability to distinguish normal cells—self—from abnormal or infected ones is referred to as "education." Avoidance of killing normal cells is referred to as "self-tolerance."
By developing a stronger understanding of how natural killer cells go about their job of killing their compromised targets, scientists gain keener insight into the role of a type of white blood cell—formally called a large granular lymphocyte.
The innate system differs from the adaptive—also called the acquired—immune system, which is composed of the highly specialized lymphocytes known as B cells and T cells. The adaptive immune system is acquired gradually and is endowed with memory—the capacity to recall an invader that has infiltrated the body in the past.
B and T cells are known for their specificity, and B cells are producers of disease-fighting antibodies. Natural killers, by contrast, use blunt force strength to rid the body of disease, but that strength relies on demonstrably sophisticated molecular mechanisms to get the job done.
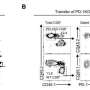
"We found that natural killer cell tolerance and education were determined by the subcellular localization of the tyrosine phosphatase, SHP-1," noted Schmied, a physician-scientist in the department of hematology and regenerative medicine at the Karolinska Institutet. As lead author of a paper titled, SHP-1 localization to the activating immune synapse promotes NK tolerance in MHC class I deficiency," Schmied emphasized that "location matters for killer cells."
Using animal models, Schmied and colleagues compared uneducated and educated cells and found that education altered the location of SHP-1. The animal model research also demonstrated that during education, inhibitory receptors sequestered SHP-1. This finding revealed how SHP-1 facilitates target cell killing.
"In mice lacking MHC-I molecules, uneducated, self-tolerant natural killer cells showed accumulation of SHP-1 in the activating immune synapse, where it co-localized with F-actin and the signaling adaptor protein," Schmied wrote.
During NK cell education, signaling through inhibitory receptors ensures that natural killer cells both tolerate normal cells and respond to molecules on cells that should be destroyed. Natural killer cell activation depends on the balance in signaling between activating receptors, which recognize proteins that are present on stressed cells, and inhibitory receptors, which recognize protein markers on healthy cells and other determinants of tolerance.
More information: Laurent Schmied et al, SHP-1 localization to the activating immune synapse promotes NK cell tolerance in MHC class I deficiency, Science Signaling (2023). DOI: 10.1126/scisignal.abq0752
© 2023 Science X Network