This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A CAR T cell therapy for advanced ovarian cancer developed
There are currently few effective treatment options for patients with recurrent ovarian cancer and other solid tumors, but City of Hope researchers are trying to change that.
Researchers with City of Hope, one of the largest cancer research and treatment organizations in the nation, have published preclinical research in Nature Communications demonstrating that a chimeric antigen receptor (CAR)-engineered T cell therapy worked against ovarian cancer in the laboratory and in preclinical models.
"City of Hope's research helped develop CAR T cell therapies for blood cancers, and these patients are now seeing long-term benefits from the therapy, but we can't stop there," said Saul Priceman, Ph.D., associate professor in the Department of Hematology & Hematopoietic Cell Transplantation and associate director of Translational Sciences & Technologies in the T Cell Therapeutics Research Laboratories at City of Hope. "The next frontier is solid tumors, and City of Hope is taking on that challenge."
The therapy is currently in a first-in-human Phase 1 trial at City of Hope for patients with advanced epithelial ovarian cancer who have already received platinum-based chemotherapy. The trial, led by Lorna Rodriguez-Rodriguez, M.D., Ph.D., City of Hope professor in its Division of Gynecologic Oncology, Department of Surgery, is testing the therapy's safety, side effects and activity of the therapy in patients. The trial is currently enrolling patients for treatment.
Developing a CAR T cell therapy for solid tumors is particularly challenging because the therapy needs to first reach the solid tumor and then survive in a harsh microenvironment that is filled with cancer cells and other cells that prevent attack by the CAR T cells. But Priceman and his team have made significant progress in overcoming these challenges.
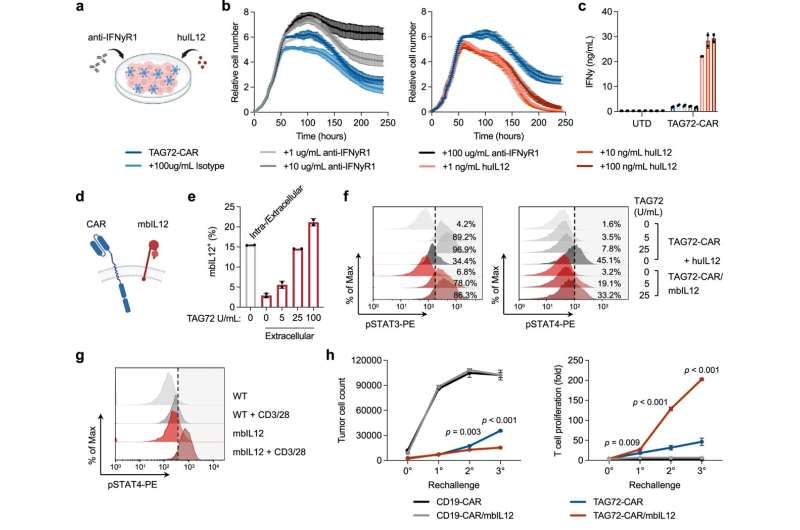
The team's most recent research found that a CAR T cell therapy targeting TAG72, a target found on the surface of ovarian cancer cells, eradicates cancer cells in mouse models.
"What's exciting about this is that TAG72 is also found on other cancer cells, including pancreatic, colorectal, breast and brain tumors, so if the clinical trial in ovarian does well, we can investigate expanding this to other patients," he added.
CAR T cell therapy involves taking a patient's T cells, a white blood cell that helps fight disease, from the bloodstream. T cells are then reprogrammed with a CAR in a City of Hope laboratory to recognize and attack a specific cancer-causing protein, such as TAG72, and reintroduced into the patient's bloodstream. CAR T cells should then eradicate cancer cells. Patients are closely monitored for any side effects.
Priceman and his team also found that by adding the cytokine Interleukin-12 (IL-12), a protein that sends signals to the immune system, to the CAR T cell therapy, the treatment worked more effectively against cancer cells in the lab.
The co-first authors Eric Hee Jun Lee and John P. Murad, Ph.D., along with the rest of the team, showed that IL-12 also enabled the T cells to both fight the cancer and leave the tumor area, enter the bloodstream and target other cancer cells around the body. Priceman noted that IL-12 is not currently part of the current Phase 1 clinical trial.
The preclinical research also found that delivering the CAR T cell therapy via an injection where the cancer is located, regionally, is also effective in enabling CAR T cells to target cancer elsewhere. This technology allows for both safety and improved anti-tumor activity in several cancer types tested to date.
"This therapy has been years in the making at City of Hope, so we are excited to finally see it in patients whose cancer is advanced and are in need of more treatments," Priceman added.
More information: Eric Hee Jun Lee et al, Antigen-dependent IL-12 signaling in CAR T cells promotes regional to systemic disease targeting, Nature Communications (2023). DOI: 10.1038/s41467-023-40115-1