This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
GKA improves glucose tolerance and induces hepatic lipid accumulation in mice with diet-induced obesity
Obesity is a major risk factor for metabolic disorders including non-alcoholic fatty liver disease and type 2 diabetes. It has been reported that non-alcoholic fatty liver disease doubles the likelihood of developing type 2 diabetes, independent of obesity and other metabolic risk factors.
Furthermore, approximately one-fifth of the global population suffers from non-alcoholic fatty liver disease, and 56% of these individuals have been diagnosed with type 2 diabetes. The number of patients diagnosed with both conditions is expected to rise continuously.
Recently, glucokinase activators (GKAs) have emerged as a breakthrough in treating type 2 diabetes. Marketed drugs such as dorzagliatin have proven effective in lowering blood glucose levels. However, GKAs may disrupt lipid metabolism, leading to fat accumulation in the liver.
Consequently, more research is required to establish the safety of GKAs in type 2 diabetes patients who also have non-alcoholic fatty liver disease. Additionally, the link between hepatic glucokinase activation and the endoplasmic reticulum stress response remains ambiguous. Further studies are needed to clarify this relationship.
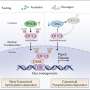
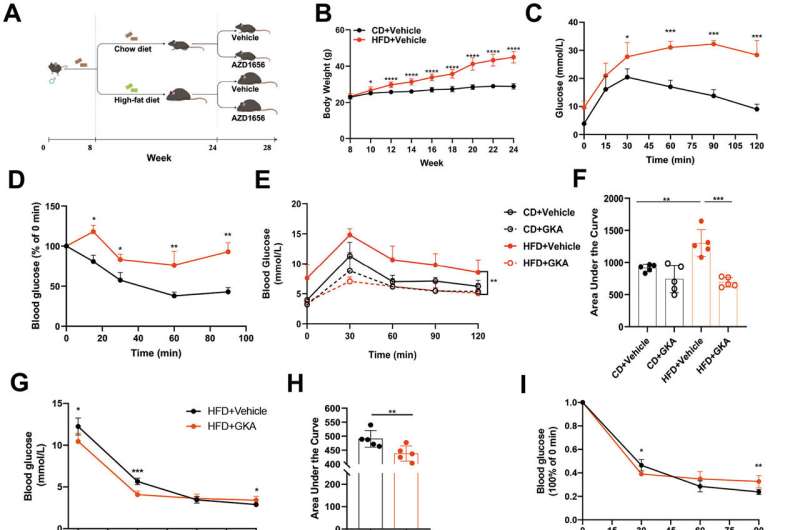
In a study published in Liver Research, a research team in China found that GKAs improved glucose tolerance and insulin sensitivity. However, GKAs also induced hepatic lipid accumulation by increasing lipogenic gene expression, which subsequently activated the hepatic PERK-UPR signaling pathway.
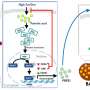
"We established a mouse model with high-fat diet-induced obesity to study the impact of GKA treatment on glucose and lipid metabolism in obese mice. We then evaluated the effect of GKA treatment on glucose metabolism in diet-induced obese mice using glucose and insulin tolerance tests," explained Nan Cai, lead author of the author.
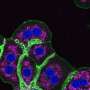
The team's findings indicated that GKA enhanced glucose tolerance by improving both islet β cell function and insulin signaling. Additionally, GKA exacerbated hepatic lipid accumulation in diet-induced obese mice, as demonstrated by hematoxylin and eosin staining, Oil Red O staining, and transmission electron microscopy. This accumulation induced hepatic pathological changes.
Overall, the study illustrated that while glucokinase activation improves glucose tolerance in mice with diet-induced obesity, it also induces hepatic lipid accumulation that activates the PERK-UPR pathway. The findings provide a theoretical basis and reference for the application of GKAs in personalized treatment of chronic diseases such as type 2 diabetes and non-alcoholic fatty liver disease.
More information: Nan Cai et al, Glucokinase activator improves glucose tolerance and induces hepatic lipid accumulation in mice with diet-induced obesity, Liver Research (2023). DOI: 10.1016/j.livres.2023.05.003