This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
'Humanized' liver in mice reveals roots of chronic diseases
Yale researchers have created a functional "humanized" liver in living mice that will help scientists find human-specific mechanisms for regulating cholesterol levels and potentially for treating chronic liver diseases afflicting tens of millions of people in the United States. The findings were published in the journal Cell.
Chronic liver diseases such as alcoholic and non-alcoholic liver disease, cancer, viral hepatitis, fibrosis, and cancer affect more than 1.5 billion people worldwide. In the U.S., an estimated 30 to 40% of the population has been diagnosed with non-alcoholic fatty liver disease alone. Yet liver disease has been difficult to study in animal models. The livers of mice, for instance, perform different functions than those of humans.
"Inside the liver multiple human cell types talk in their own language," said senior author Richard Flavell, Sterling Professor of Immunobiology at Yale School of Medicine and investigator for the Howard Hughes Medical Institute. "Mouse and human cells talk in different languages, but we have enabled human liver cells to speak in their own language within living mice."
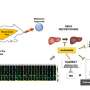
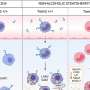
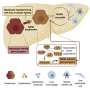
For the study, a team of scientists led by Eleanna Kaffe, an associate research scientist in Flavell's Lab, used progenitor stem cells and mature cells known as hepatocytes from a human liver to create a complete human liver in a mouse model. The humanized liver, researchers said, developed into similar size-adjusted shape and carried out similar cellular functions as a healthy human liver. The cellular functions in the humanized liver could also be manipulated to mimic human fibrosis and non-alcoholic fatty liver disease, the researchers report.
The researchers also found that essential liver metabolism is controlled by activity in endothelial cells, which line blood vessels that feed the liver. Those endothelial cells, they said, secrete a signaling molecule called Wnt which regulates cholesterol transport to hepatocytes for the synthesis of bile acid. The transport of cholesterol to hepatocytes is an important mechanism that reduces excess blood cholesterol levels in humans.
According to the researchers, the humanized liver model can be used immediately by drug companies seeking to assess safety of experimental drugs designed to treat chronic diseases.
"However, our long-term goal is to find ways to predict, prevent, and treat all liver diseases, which take such a huge toll on individuals," the authors said.
More information: Richard A. Flavell, Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions, Cell (2023). DOI: 10.1016/j.cell.2023.07.017. www.cell.com/cell/fulltext/S0092-8674(23)00795-X