This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Inhalation drug may prevent severe pneumonia
Overly active immune cells are often behind lung damage in diseases such as COVID-19. Researchers at the Technical University of Munich (TUM) have developed an RNA agent for a lung spray that slows the activity of these cells, known as macrophages. A new, sugar-based transport mechanism is especially effective in bringing the therapeutic to its target.
The team led by Stefan Engelhardt, Professor of Pharmakology and Toxikology, has developed an RNA-based active ingredient called RCS-21 to prevent severe lung inflammation and fibrosis, i.e., scarring of the lung tissue, for example in SARS-CoV2 infections.
In the cell, RCS-21 stops the activity of the molecule microRNA 21. This nucleic acid, which Engelhardt and his team have been researching for a long time, is one of the triggers for the excessive activity of macrophages in severe lung infections.
Drug docks onto sugar receptors
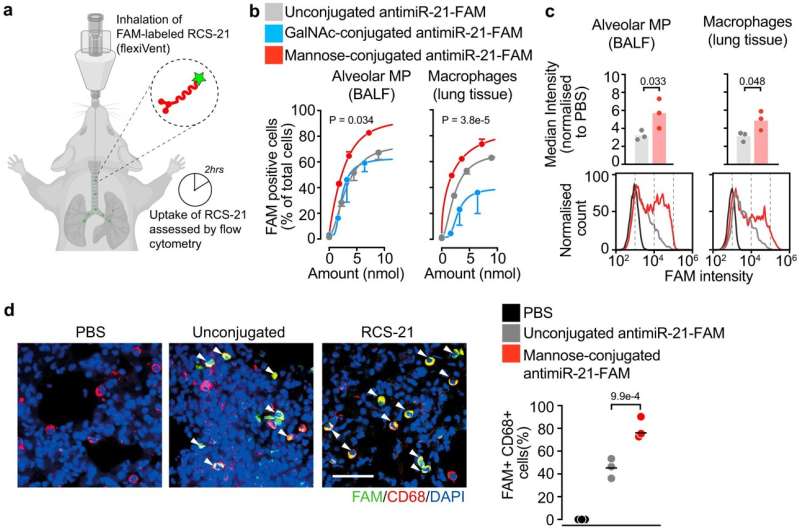
Publishing in the scientific journal Nature Communications, the team describes how the active substance RCS-21 is delivered to its target particularly effectively via an inhaler. To do this, the researchers took advantage of a special feature of macrophages. These scavenger cells are also present in large numbers in the healthy lung. There, they perform the important task of destroying bacteria and fungal spores as quickly as possible.
The macrophages identify their targets among other things based on complex sugar molecules on the surface of the invaders. "We have determined in single cell analyses that the corresponding sugar receptors are, on the one hand, among the most common receptors on macrophages," says Stefan Engelhardt. "On the other hand, the receptors are, in a sense, a unique feature of macrophages—they hardly occur anywhere else."
Therefore team coupled its active ingredient to a sugar molecule, or more precisely, to trimannose. This approach had so far only been pursued with chemically less complex active ingredients. Studies with mice produced clear results. "When the drug was administered as a spray, macrophages took up the active ingredient significantly better than without sugar molecules. In contrast, other cell types even outright exclude the molecules," says Christina Beck, first author of the article together with Deepak Ramanujam.
Active substance successfully tested
In experiments with mice, RCS-21 ensured that microRNA 21 was reduced by more than half compared to control animals. Fibrosis and inflammation were also significantly reduced after treatment. Increased microRNA-21 activity was also stopped by treatment with RCS-21 in samples of human lung tissue infected with the SARS-CoV-2 coronavirus in the laboratory.
Studies to prove the drug's safety are already underway, the first clinical trials in humans are targeted for 2024. Responsibility lies with RNATICS, a TUM spin-off.
RNATICS co-founder Stefan Engelhardt sees great potential in the mannose technology. "We were able to show that nucleic acid-based active substances can be used in a very targeted manner, at least in the lungs. This technology opens up a wide field for the development of novel RNA-based drugs. I expect a lot to happen in this area in the next few years."
More information: Christina Beck et al, Trimannose-coupled antimiR-21 for macrophage-targeted inhalation treatment of acute inflammatory lung damage, Nature Communications (2023). DOI: 10.1038/s41467-023-40185-1