This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Assessing the mechanisms for liver fibrosis
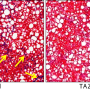
Chronic liver injury results in the development of liver fibrosis in approximately 25% of individuals in response to diverse insults, and several aspects of the development of liver injury appear to be common to a variety of initiating factors. In contrast, others are specific to individual types of injury. Among all the hepatic changes due to metabolic syndrome, understanding the development of fibrosis is a priority as it is the strongest predictor of mortality.
Metabolic syndrome is an umbrella term characterized by obesity and insulin resistance and is the underlying driving force for non-alcoholic steatohepatitis (NASH). The increased prevalence of obesity has significantly increased the importance of liver fibrosis in the setting of metabolic syndrome and NASH.
In a new paper published in eGastroenterology, Professor Wajahat Mejel from Yale University reviewed the common features of the liver fibrotic response and examined the role of the metabolic syndrome in individual mechanisms of liver fibrosis.
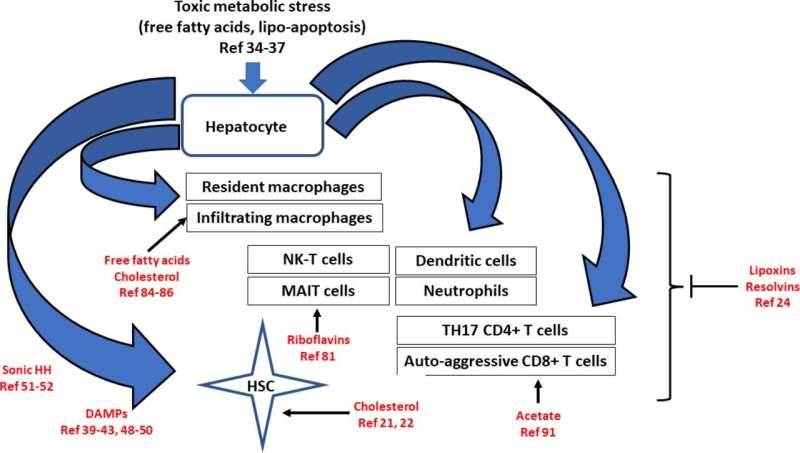
Liver fibrosis is when hepatic stellate cells (HSCs) are converted into myofibroblasts. Myofibroblasts are responsible for producing extracellular matrix, which is the main component of scar tissue. HSCs can be activated by various factors, including injury to the liver cells, inflammation, and exposure to toxins.
Once activated, HSCs undergo several changes, including increased proliferation, production of growth factors and pro-inflammatory cytokines, and secretion of matrix degradation enzymes. These changes lead to the deposition of scar tissue, which can eventually lead to liver failure.
Recent studies have shown that HSCs can also be activated without liver injury and that these cells can play a role in developing chronic liver disease. Understanding the mechanisms of HSC activation is essential for developing new treatments for liver fibrosis.
CD8+ T cells play a significant role in metabolic syndrome-associated liver injury. These cells activate HSCs and induce insulin resistance. In lean NASH models, CD8+ T cells do not seem to significantly affect liver injury or HSC activation. However, in obese NASH models, CD8+ T cells are reduced, associated with increased liver injury and HSC activation.
A subset of CD8+ T cells in NASH models have a tissue-resident phenotype and can kill hepatocytes in a purinergic receptor-dependent but MHCI-independent manner. These cells can also induce HSC death via a classical Fas/Fas-L pathway. The net effect of CD8+ T cells on liver fibrosis may depend on the stage of the disease. They may have a NASH-driving role in early disease by increasing insulin resistance and hepatocyte damage. In later fibrosis stages, they may have a fibrosis resolution effect by killing HSCs.
The development of NASH is a complex process involving genetic and environmental factors. The genetic contribution to NASH is estimated to be between 20 and 70 percent, but this number may vary depending on the study population and the definition of NASH. The environmental factors contributing to NASH include obesity, metabolic syndrome, and certain medications.
The assessment of the genetic contribution to fibrosis in NASH is limited because most studies have focused on the genetic contribution to the development of NAFLD or NASH rather than fibrosis. Also, most of the data on the genetics of NASH comes from European populations, so it needs to be clarified how generalizable these findings are to other people.
The development of diabetes mellitus (DM) and nonalcoholic steatohepatitis (NASH) share many etiological factors, such as overnutrition and high carbohydrate intake. Both conditions also share the critical pathophysiological feature of total body insulin resistance. It is, therefore, not surprising that there is a greater prevalence of NASH in the DM population.
From 2014 to 2017, the global presence of NAFLD in a DM population was 55 percent, while for NASH was 37 percent, and the estimated prevalence of advanced fibrosis among the NAFLD population was 17 percent. Other studies have also identified a greater than two-fold increase in cirrhosis and complications of cirrhosis in patients with DM.
These factors suggest that features of DM may be driving liver fibrosis. The connection between DM and fibrosis also extends beyond NASH and the liver, as DM is well known to result in cardiac, renal, and pulmonary fibrosis, all organs which lack the parenchymal pathology of NASH, such as steatosis.
White adipose tissue (WAT) also undergoes fibrosis in the setting of obesity and metabolic syndrome. This contrasts with the liver, where fibrosis is typically driven by inflammation. Fibrosis is thought to be caused by inadequate angiogenesis in WAT. This leads to the hypoxic death of adipocytes and subsequent inflammation.
This process can physically constrain the growth of WAT, which may redirect nutrients to other organs, including the liver. The implications of WAT fibrosis for liver fibrosis are unclear, but there is evidence that it can contribute to hepatic steatosis.
Liver fibrosis is a common feature of all types of chronic liver injury, but metabolic syndrome-driven liver fibrosis has unique aspects. In metabolic syndrome, there are many points of initiation and progression, including hepatocyte damage, innate immune activation, and adaptive immune activation. The cell-cell interactions in established liver fibrosis in NASH are predominantly autocrine loops, with a minimal role for hepatocyte-HSC interactions. Understanding these cellular interactions is critical to developing therapies for liver fibrosis.
More information: Wajahat Mejel et al, Mechanisms of liver fibrosis in metabolic syndrome, eGastroenterology (2023). DOI: 10.1136/egastro-2023-100015