This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel mouse model may help to develop treatment for neurobrucellosis
Neurobrucellosis is a severe long-term complication of brucellosis, one of the most common bacterial zoonotic diseases. Scientists have now developed a novel animal model that indicates that innate lymphoid cells and interferons play an important role in limiting arthritis, meningitis, and neurologic complications during Brucellamelitensis infection. According to a new report in The American Journal of Pathology, this may help understand how neurobrucellosis develops and consequently help explore potential therapies.
Brucellosis is one of the most common bacterial zoonotic diseases in the world, causing significant agricultural and public health problems especially in endemic areas such as the Mediterranean Basin, Middle East, and Central Asia. In animals, it presents as late-term abortion in affected livestock species and causes devastating financial losses. In humans, it can infect and cause disease in almost any organ or organ system.
High rates of human disease correlate with lambing season in endemic countries because the disease is transmitted to humans through the ingestion of unpasteurized dairy products or through direct contact (ingestion, inhalation, and wound infection) with contaminated tissues. Brucellosis accounts for more than 500,000 recorded human cases per year, but many believe the actual number of annual infections is 10 to 20 times higher because most cases go undiagnosed.
Lead investigator Jerod A. Skyberg, Ph.D., Department of Veterinary Pathobiology, College of Veterinary Medicine and Laboratory for Infectious Disease Research, University of Missouri, Columbia, explains: "The disease in humans is characterized by an undulating fever and systemic symptoms, including lethargy, chills, arthralgia, and headaches."
"The most morbid complication is neurobrucellosis, in which neural tissue is damaged either directly through bacterial disruption or indirectly via severe immune inflammatory responses. The most severely affected patients can develop life-long neurologic consequences."
The researchers found that innate lymphoid cells and interferons play an important role in defense against central nervous system infection by Brucella and in doing so, have developed novel mouse models for neurobrucellosis to study this further.
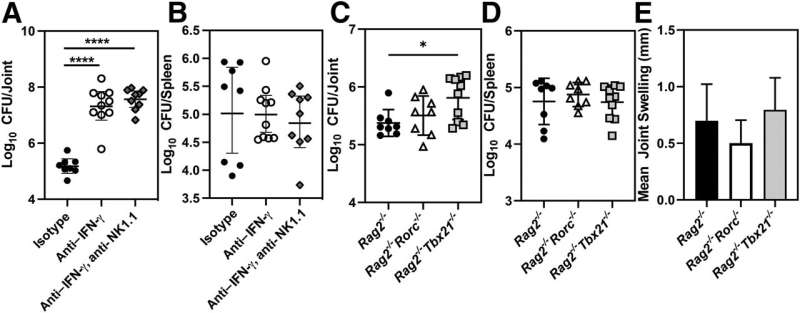
![Innate lymphoid cells prevent development of meningitis following pulmonary infection. Rag2−/−/Il2rg−/− mice were infected intranasally with 105 colony-forming units of Brucella melitensis. At the development of neurologic complications, or at the conclusion of the study (70 days after infection), brains were collected and transversely sectioned for histopathology. A: Slides were stained with hematoxylin and eosin, and representative images [4× objective (left panels) and 10× objective (right panels)] of naïve and infected sections from the caudal cerebral cortex are shown. The arrows indicate lesions of inflammatory cell infiltrate, and the circle indicates perivascular cuffing. B and C: Immunohistochemistry was performed on serial sections to detect myeloperoxidase (MPO; B) or Brucella antigen (C). B and C: Representative images (10× objective) of rostral cerebrum (left panels), cerebellum (middle panels), and caudal cerebrum (right panels) indicate infiltration of MPO-positive cells (B) and Brucella (C) into the meninges. n = 10 Rag2−/−/Il2rg−/− mice (A–C). Credit: The American Journal of Pathology (2023). DOI: 10.1016/j.ajpath.2023.05.006 Novel mouse model may help to develop treatment for neurobrucellosis](https://scx1.b-cdn.net/csz/news/800a/2023/novel-mouse-model-may-1.jpg)
Investigators studied the role of innate lymphoid cells (ILCs) and interferons in the pathogenesis of focal brucellosis. After performing footpad infections with Brucella to induce arthritis in mice, they switched to an intranasal infection route to mimic a natural route of infection more closely. In the course of these studies, they observed neurologic signs in mice lacking ILCs and interferons.
ILCs limited articular complications of brucellosis, regardless of the route of infection. In addition, mice lacking ILCs developed neurologic complications of brucellosis including meningitis and colonization of the brain by Brucella. Brucella infection caused a marked upregulation of genes associated with interferon responses and a downregulation of genes associated with neurologic function in the brains of infected mice. Mice lacking interferon signaling also rapidly developed clinical signs of neurobrucellosis and exhibited hippocampal neuronal loss.
Lead author Charles R. Moley, DVM, Department of Veterinary Pathobiology, College of Veterinary Medicine and Laboratory for Infectious Disease Research, University of Missouri, Columbia notes, "Prior to these observations there had been no experimental animal model for neurobrucellosis, so seeing these signs develop was quite exciting."
"We were also hoping to be able to visualize the bacteria within the brain via immunohistochemistry; however, we were surprised at the extent of Brucella antigen within the meninges and ventricles of the infected mice. In addition, while type I interferon signaling had previously been reported to be deleterious to control of Brucella infection, here we found that type I interferon signaling limited both colonization of the brain and neuronal loss in response to Brucella infection."
Collectively, these findings indicate ILCs and interferons play an important role in limiting both arthritis and meningitis during Brucella infection. In addition, this work details the first mouse models of neurobrucellosis in which Brucella colonizes the brain, induces inflammation, and impairs neurologic function. The investigators observe that these models could be used to investigate multiple aspects of neurobrucellosis such as invasion of the brain by Brucella or therapeutics for treatment of neurologic complications of brucellosis.
Dr. Skyberg and Dr. Moley conclude, "Considering that neurobrucellosis is the most morbid complication caused by this bacterium, learning more about its development, effects on the immune system, and potential therapies to counteract damage to the host central nervous system may benefit patients worldwide."
More information: Charles R. Moley et al, Innate Lymphoid Cells and Interferons Limit Neurologic and Articular Complications of Brucellosis, The American Journal of Pathology (2023). DOI: 10.1016/j.ajpath.2023.05.006