This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists identify key mechanism for nerve cell growth critical for three-dimensional vision
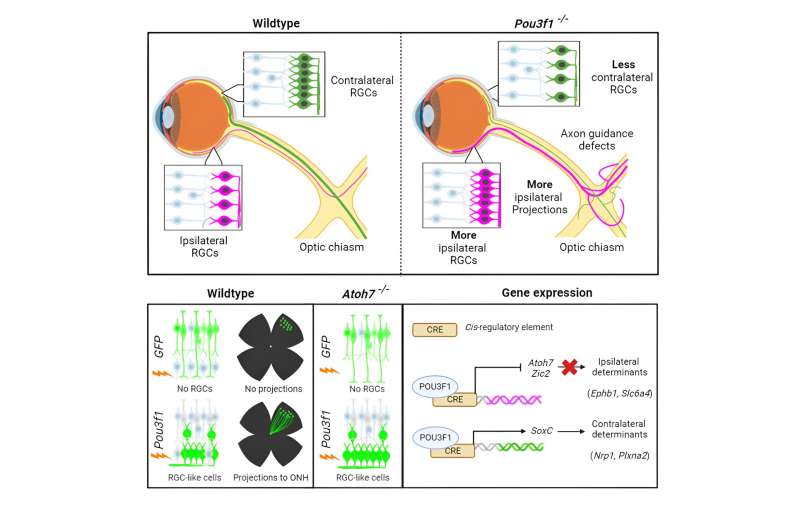
Scientists in Montreal have identified a key mechanism involved in the growth of nerve cells that are critical to mediate binocular vision, which allows people to see the world in three dimensions.
The work was led by Université de Montréal medical professor Michel Cayouette at the Montreal Clinical Research Institute's cellular neurobiology research unit. It was published this month in Cell Reports.
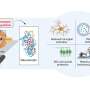
To see the world in 3D, our eyes look at an object from two different parts of the retina, a thin layer tissue at the back of the eyes that transforms light into biological signals. The overlap between these two fields of vision allows us to determine the depth, distance and speed of an object and make fast, sometimes lifesavings decisions. Crucial for this process is the proper growth of nerves from the eye to the brain.
When these nerve cells—called retinal ganglion cells—send projections to the brain via the optic nerve, they either remain on the same side or cross over to the other half of the brain. It is the balance of these projections that allows us to see the world in 3D, but until now how exactly this is controlled has remained poorly understood.
The work of Cayouette and his colleagues marks an important advance toward solving this mystery.
A new gene is discovered
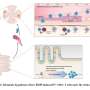
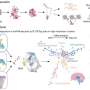
In their study, the scientists identified a gene called Pou3f1.
It acts as a major regulator controlling the expression of dozens of other genes, which together generate the full instructions to ensure retinal ganglion cells send projections that cross to the opposite hemisphere of the brain.
The researchers showed that expression of Pou3f1 in retinal stem cells is sufficient to force them to become retinal ganglion cells sending projections to the optic nerve.
"Our work identifies Pou3f1 as a critical regulator of processes underlying binocular vision in mammals and as a potential candidate for the regeneration and repair of the visual system," said Thomas Brown, a doctoral student in Cayouette's lab and the study's co-first author with former student Michel Fries.
"Nerves are roads for information, and if they cannot send information to the appropriate area in the brain, then it leads to serious problems, as observed in blinding eye diseases like glaucoma," added Christine Jolicoeur, a senior research assistant in the team and co-author of the study.
"Our work helps understand how the roadmap of visual information is constructed and sheds light on how nerves reach the right area of the brain, which is essential information to help develop regenerative approaches for various neurodegenerative diseases," Cayouette concluded.
More information: Michel Fries et al, Pou3f1 orchestrates a gene regulatory network controlling contralateral retinogeniculate projections, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112985