This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Team discovers mechanisms of rare disease that is often fatal if Epstein-Barr virus infects individual

A University of Ottawa-led research team has published new research that advances a quest to understand an ultra-rare disease that's found almost exclusively in boys. The study was published in PLOS Pathogens.
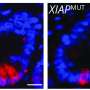
XLP-2 is a genetic X-linked lymphoproliferative disease first described in 2006. It typically has severe complications among patients who become infected with the Epstein-Barr virus, an exceedingly common virus that infects most people without problems in their teenage years or young adulthood. But when the few individuals with XLP-2 encounter the Epstein-Barr virus the experience is often fatal because it results in immunodeficiency—a derailing of the immune system.
The team aimed to examine how the inactivation of a protein-coding gene called XIAP triggers this immunodeficiency, according to senior author Dr. Subash Sad, whose uOttawa Faculty of Medicine lab studies the mechanisms that maintain a healthy immune system and prevent the development of inflammatory diseases.
"How inactivation of XIAP results in immunodeficiency was not clear. It was speculated that an inactivation of XIAP impacts T cells directly. However, our work demonstrates that this is not entirely true. We showed inactivation of XIAP impacts the survival of T cells through direct and indirect effects which comprehensively result in a state of immunodeficiency," says Dr. Sad, a cellular immunologist and professor in the Faculty's Department of Biochemistry, Microbiology and Immunology.
First author Parva Thakkar and the other researchers used recombinant bacteria and T cell transgenic cells to monitor the impact of XIAP on the differentiation and memory development of T cells following infection. Most of the experiments were done in vivo with a mouse model. Other mechanistic experiments were done in various cells of the immune system.
Ultimately, the team's efforts revealed two underlying mechanisms: Inactivation of XIAP results in poor expression of Interleukin-6 (IL-6) which compromises the proliferation of activated T cells, and inactivation of XIAP compromises the ability of activated T cells to survive long-term.
The consequence of this phenomenon is that memory T cells, which patrol the body and are induced during prior infections or vaccinations, survive poorly if there is an inactivating mutation in XIAP. This explains the reasons behind immunodeficiency in XLP-2 patients, Dr. Sad says.
As the team explores questions suggested by this study, they are interested in examining downstream mechanisms and pathways that can be targeted for therapy. They are also interested in working with patient samples. Dr. Sad encourages families with such mutations to get in contact with CHEO, an institutional partner of the uOttawa Faculty of Medicine.
More information: Parva Thakker et al, XIAP promotes the expansion and limits the contraction of CD8 T cell response through cell extrinsic and intrinsic mechanisms respectively, PLOS Pathogens (2023). DOI: 10.1371/journal.ppat.1011455