This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Trial demonstrates potential of acoramidis for transthyretin amyloid cardiomyopathy

Acoramidis improves outcomes in patients with transthyretin amyloid cardiomyopathy (ATTR-CM) compared with placebo, according to late breaking research presented in a Hot Line session today at ESC Congress 2023.
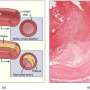
ATTR-CM is a rare, progressive, and fatal disease characterized by the accumulation of misfolded transthyretin protein in the heart. It causes an infiltrative, restrictive cardiomyopathy resulting in clinical heart failure, usually with preserved ejection fraction. Previously, the ATTR-ACT trial of tafamidis in ATTR-CM demonstrated a reduction in all-cause mortality, cardiovascular-related hospitalization and declines in functional capacity by 6-minute walk distance (6MWD) and quality of life assessed by the Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OS), as compared with placebo.
Acoramidis has been shown to be a superior stabilizer of transthyretin as compared with tafamidis in vitro, and a phase 2 study in ATTR-CM patients with symptomatic heart failure suggested that it had the potential to be a safe and effective therapy for such patients.
ATTRibute-CM was a multinational, randomized, double-blind, placebo-controlled phase 3 trial evaluating the efficacy and safety of acoramidis in patients with ATTR-CM. Eligible patients with wild-type or variant symptomatic ATTR-CM were randomly allocated in a 2:1 ratio to oral acoramidis 800 mg twice daily or placebo for 30 months. Participants in both arms had the option of initiating open-label, commercially available tafamidis after 12 months in the study. Patients were invited to participate in an open-label, long term extension study of acoramidis if they completed the 30-month ATTRibute-CM study.
The primary endpoint, analyzed at 30 months, was a hierarchical analysis by the Finklestein-Schoenfeld method of all-cause mortality, cardiovascular-related hospitalization, NT-proBNP, and 6MWD. Secondary endpoints included the components of the primary endpoint, KCCQ-OS, and serum transthyretin levels.
A total of 632 patients with ATTR-CM were randomized. The median age was 78 years, 90% of participants were male, and 10% were variant TTR carriers. Most participants had either New York Heart Association Class II (72.0%) or Class III (17.2%) symptoms. The primary hierarchical endpoint analysis was highly statistically significant, resulting in a win ratio of 1.8 (95% confidence interval [CI] 1.4 to 2.2; p<0.0001).
There was a consistent, positive treatment effect across all components of the primary endpoint analysis, including a numerical reduction in all-cause mortality, with an absolute risk reduction (ARR) of 6.4%, relative risk reduction (RRR) of 25%, and hazard ratio (HR) of 0.772 (95% confidence interval [CI] 0.542 to 1.102; p=0.15). The cumulative frequency of cardiovascular-related hospitalizations was reduced by about half in the acoramidis arm, for an ARR of 0.226 cardiovascular-related hospitalizations/year and RRR of 50.4% (95% CI 30.5% to 64.5%; p<0.0001).
Change from baseline in NT-proBNP was lower in the acoramidis arm than in the placebo arm at month 30 (ratio of adjusted geometric mean fold-change 0.529; 95% CI 0.463 to 0.604; p<0.0001) and the decline in change from baseline in 6MWD was reduced with a least squares mean difference of 39.64 m at month 30 in favour of acoramidis (95% CI 21.07 to 58.22; p<0.0001).
Analysis of the remaining secondary endpoints demonstrated that acoramidis preserved quality of life as assessed by change from baseline in KCCQ-OS compared to placebo with a least squares mean difference at month 30 of 9.94 points (95% CI 5.97 to 13.91; p<0.0001), and resulted in greater increases in change from baseline in serum transthyretin, with a least squares mean difference at month 30 of 7.1 mg/dL (95% CI 5.79 to 8.40; p<0.0001), reflecting transthyretin stabilisation in vivo. Treatment with acoramidis was generally well-tolerated.
Principal investigator Professor Julian Gillmore of University College London at the Royal Free London NHS Foundation Trust, UK, said, "Despite the challenges imposed by the COVID-19 pandemic, we were able to enroll patients largely from countries without access to tafamidis, such as the UK. The trial showed that in patients with ATTR-CM, acoramidis was consistently associated with clinical benefits as reflected in the primary hierarchical analysis, with 58% of the win ratio ties broken by all-cause mortality and cardiovascular-related hospitalizations, and an overall win ratio of 1.8 that was highly statistically significant.
"The positive treatment effect was consistent across components of the primary endpoint, among key clinical subgroups, and across key secondary endpoints. Acoramidis was more effective in preserving both functional capacity and quality of life, and increased circulating transthyretin levels, compared with placebo. Acoramidis has the potential to be an effective and safe alternative to tafamidis for the treatment of ATTR-CM."