This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers identify driver of inflammatory bowel disease
Researchers at UT Southwestern Medical Center have discovered an intracellular mechanism that converts protective intestinal cells into disease-driving pathogenic cells, a finding that could lead to improved treatments for patients with inflammatory bowel disease (IBD).
The research, published in Nature Communications, defines a mechanism by which healthy cells in the gut—known as Th17 cells—are compromised in patients with IBD, producing inflammation that causes abdominal pain, bloating, and other symptoms, along with potentially serious long-term complications.
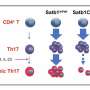
"Th17 cells are essential for maintaining the integrity of the intestinal barrier and protecting against bacteria and viruses. But in patients with IBD, they become pathogenic, creating the inflammation that underlies the disease," said study leader Venuprasad Poojary, Ph.D., Associate Professor of Internal Medicine and Immunology in the Division of Digestive and Liver Diseases. "Through our research, we now have a better understanding of the intracellular process that converts protective Th17 cells into disease-generating, pathogenic, inflammatory Th17 cells."
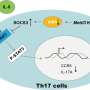
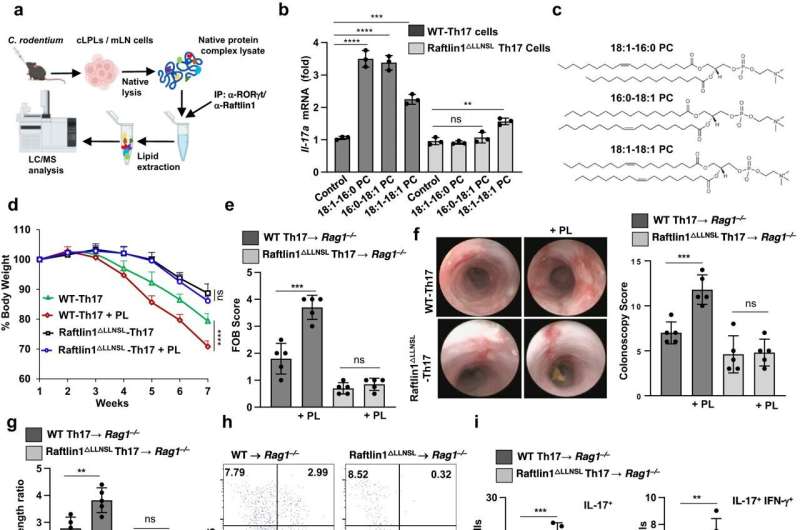
The study in mice found that a lipid-interacting protein called Raftlin1 binds to RORγt, a transcription factor in Th17 cells, as IBD develops. Once in place, Raftlin1 attracts phospholipids in the body to combine with the RORγt, eventually turning Th17 cells pathogenic.
The research builds on other cellular studies related to IBD conducted by Dr. Poojary and his lab.
"Identifying the role of Raftlin1 in facilitating the binding of phospholipids to RORγt is a major step forward in our understanding of the diverse and opposing functions of Th17 cells," Dr. Poojary said. "It's an important finding because Th17-targeting therapies have shown promising results with some autoimmune diseases. There is a great need for new pharmaceuticals that can effectively treat IBD, since about a third of patients with the disease don't respond to existing treatments."
More than 3 million Americans suffer from IBD, with Crohn's disease and ulcerative colitis the most common forms. Although the disease is similar to irritable bowel syndrome (IBS), the symptoms of patients with IBD are caused by inflammation in the GI tract, diagnosed through a colonoscopy. That inflammation can permanently damage the intestines, create severe complications throughout the body, and put patients at a higher risk of colon cancer.
"These findings could serve as a platform for therapeutic strategies to control Th17-mediated inflammation in IBD and other diseases," Dr. Poojary said.
More information: Amir Kumar Singh et al, RORγt-Raftlin1 complex regulates the pathogenicity of Th17 cells and colonic inflammation, Nature Communications (2023). DOI: 10.1038/s41467-023-40622-1