This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Minimizing fallout from explosive cell death could slow inflammation, cancer spread
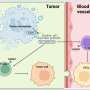
Research suggests one of the many ways cancer can spread is an explosive form of cell death known as necroptosis.
This regulated self-destruction purges cancerous and other diseased cells from the body, yet its volatile method—the cell membrane bursts, releasing inflammatory substances into nearby tissue and the bloodstream—can actually help cancer metastasize.
Now, University at Buffalo scientists have revealed the mechanism behind this cellular explosion and, crucially, how to minimize it.
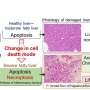
Their previous studies suggest the cell ruptures during necroptosis via a buildup of toxic fatty acids on the membrane. Now, in a recent study published in Chemical Biology, they inhibited a producer of fatty acids and other lipids, known as sterol regulatory element-binding proteins (SREBPs). This kept cells more intact and caused fewer of them to die.
"We knew lipids accumulated and we knew they were toxic," says G. Ekin Atilla-Gokcumen, Dr. Marjorie E. Winkler Distinguished Professor and associate chair in the UB Department of Chemistry, within the College of Arts and Sciences. "What we have learned now is that SREBP activation causes these toxic accumulations, and if we can target SREBP, we can reduce membrane permeabilization and necroptosis."
The research could lay the groundwork for lipid-related cancer treatment in the future, as reducing fatty acid buildup during necroptosis could reduce the release of the pro-inflammatory molecules that spread cancer.
"We hope that gaining a fundamental understanding of lipid accumulation during necroptosis could ultimately inspire the development of therapeutic strategies against lipid-related toxicities in cell death," says the study's first author, Daniel Lu, who received his chemistry Ph.D. from UB earlier this year.
The research was led by Atilla-Gokcumen, Ph.D. Other contributors were Omer Gokcumen, Ph.D., professor of biological sciences in the College of Arts and Sciences, and Laura R. Parisi, who received her chemistry Ph.D. from UB in 2019.
Figuring out the how of fatty acid buildup
Atilla-Gokcumen's lab focuses on lipids—a broad range of compounds that include fats—and how they impact cellular function.
About eight years ago, she and her team observed that human and animal cells undergoing necroptosis produced more lipids. Specifically, an unusual class of lipids known as very long chain fatty acids.
"We thought there must be a reason why," Atilla-Gokcumen says.
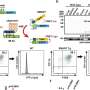
After documenting the link between very long chain fatty acids and membrane permeabilization, they turned their attention to how the acids accumulate in the first place. Using a technique called transcriptomics, they found that SREBP target genes upregulated during necroptosis, suggesting that they were responsible.
To confirm this, the scientists treated colon cancer cells with an SREBP small molecule activator and then induced necroptosis, resulting in a three-fold increase in fatty acids. Consequently, the membranes weakened even more than in regular necroptosis, shown in the release of an inflammatory marker, and more cells died.
They also infused cancer cells with betulin, which makes SREBP less active, and again induced necroptosis. This strengthened the membrane, shown by a 40% drop in propidium iodide, a fluorescent molecule that helps differentiate intact membranes and permeable ones. In addition, 85% of the betulin-treated cells survived compared to just 50% of the control group cells undergoing necroptosis.
The scientists also used other methods to turn off SREBP during necroptosis, and again found the same result: fewer fatty acids, smaller membrane rupture and better cell survival.
"This finding is crucial," Lu says. "It suggests that an upstream mechanism that regulates lipid production during necroptosis was identified for the first time."
A paradoxical role in cancer progression
Among potential cancer treatments, necroptosis is considered a double-edged sword, Atilla-Gokcumen says.
Some scientists view it as a failsafe for killing cancer cells resistant to the more common, less volatile cell death known as apoptosis. There's even some evidence that the necroptotic release of inflammatory molecules can fight cancer, as inflammation attracts immune cells to the scene.
However, inflammation can also promote cancer progression. The release of intracellular materials, such as pro-inflammatory cytokines and chemokines, can aid both tumor creation and tumor spread.
"Inflammation can initiate a cascade of events that ends up making us even more sick," Atilla-Gokcumen says.
Her lab has since written another paper, available in pre-print form on bioRxiv on how very long chain fatty acids cause membrane permeabilization. They found evidence the fatty acids serve as hotspots on the membrane that prime it for explosion.
Atilla-Gokcumen says it may be like squeezing water out of a cloth.
"It's not one huge leak that completely disintegrates and spills the guts out of the cell, if you will, but rather, there are multiple points of little explosions," she says.
More information: Daniel Lu et al, SREBP activation contributes to fatty acid accumulations in necroptosis, RSC Chemical Biology (2023). DOI: 10.1039/D2CB00172
A Apoorva J. Pradhan et al, Acylation of MLKL impacts its function in necroptosis, bioRxiv (2023). DOI: 10.1101/2023.08.19.553906