September 22, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Ranitidine study finds no elevated risk of cancer... despite finding elevated cancer rates
Research led by Seng Chan You, MD, at the Yonsei University College of Medicine, Korea, has investigated the risk of cancer associated with use of the drug ranitidine compared to other histamine-2 receptor antagonists (H2RAs) using a large-scale cohort study across multiple countries.
In the paper, "Ranitidine Use and Incident Cancer in a Multinational Cohort," published in JAMA Network Open, the researchers claim that despite contamination with a probable human carcinogen N-nitrosodimethylamine (NDMA) found in ranitidine, there was no statistically significant evidence that exposure to the drug was associated with an increased risk of cancer.
In a results statement the authors claim, "In this cohort study including 1,183,999 individuals from 11 large databases across Europe, North America, and Asia, risk of cancer among ranitidine users did not differ from users of other H2RAs. Ranitidine use was not associated with an increased risk of esophageal, stomach, or colorectal cancer, or 13 other subtypes of cancer."
The data provided in their study refutes this result statement in some significant ways. First, the actual cohort was much smaller.
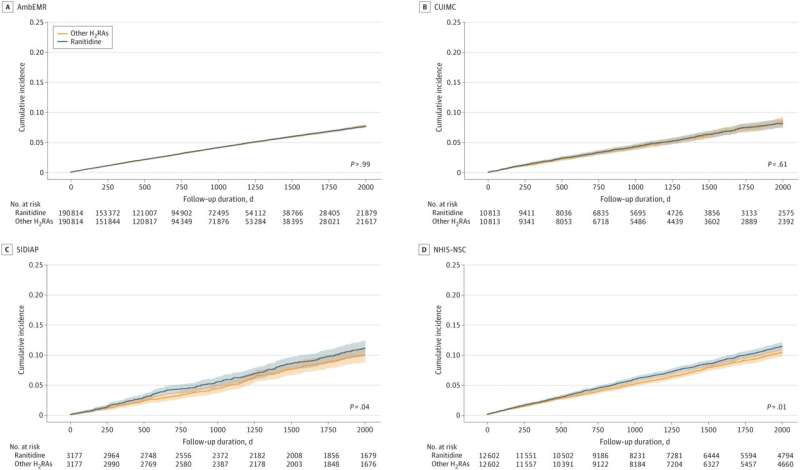
To compare ranitidine use to alternatives available within cohorts at a 1:1 ratio, the actual data used for the study's primary analysis included 434,812 individuals from four databases, half of which were exposed to ranitidine.
One of these databases, IQVIA US Ambulatory Electronic Medical Research (AmbEMR), accounts for 381,628 individuals, therefore making up the bulk of the primary analysis and has the shortest follow-up period of ranitidine users at 2.61 years.
Also included in the primary analysis were the Columbia University Irving Medical Center data warehouse (CUIMC) databases (mean follow-up of 3.6 years), NHIS-NSC, Korean National Health Insurance System-National Sample Cohort (NHIS-NSC) with a mean follow-up of 4.5 years, and Spain's Information System for Research in Primary Care (SIDIAP) with a mean 5.8-year duration follow-up.
A subgroup meta analysis was provided with the excluded cohorts and the numbers were reduced to match the lower rates of local use of alternatives to ranitidine. In the case of the U.K.'s IQVIA Medical Research Data (IMRD), the number of ranitidine users was reduced from 178,298 to just 633 individuals.
While the hazard ratio (HR) across 16 different cancers in the primary study of four cohorts over four different follow-up durations was 1.04 overall, some individual cancer types showed more significant HR.
The authors stated that ranitidine use was not associated with an increased risk of esophageal (HR 1.08) or stomach (HR 1.17) cancers, though 8% and 17% increased events seem significant.
The authors stated that ranitidine was not associated with increased risk in 13 other cancers like leukemia (HR 1.12) and gallbladder/ biliary tract (HR1.14) cancer, though 12% and 14% increases appear significant.
It is unclear how corpus uteri endometrial (HR 1.20) and Ovary (HR 1.26) cancer rates were not considered above the threshold of significant event associations, especially in such a short-term study.
It would be interesting to know the age ranges of the women with these events as NDMA can interfere with DNA replication during cell division, something more likely to be occurring in reproductive age ovary and corpus uteri tissues, but that data was excluded from the study.
Exposures to carcinogens are known to contribute to later-life disease formation even after the exposure source is removed, requiring longer term study, which is why the drug was pulled off the market in the first place.
Ranitidine and NDMA
Ranitidine, sold under the brand name Zantac, was once widely used to treat conditions like heartburn and peptic ulcer disease but was withdrawn from the market in 2020 by request of the FDA due to contamination with NDMA. The FDA investigation found factors like heat and the duration of time since ranitidine was manufactured led to greater levels of NDMA. These conditions could raise the level of NDMA in the ranitidine product above the acceptable daily intake limit.
NDMA is a DNA-damaging agent that creates 3-methyladenine, which can interfere with the copying of DNA. Damaged DNA causes mutations that drive cancer, which may take many years to develop.
Exposure to NDMA has been specifically linked to childhood cancer rates in a real-world scenario. The Olin Corporation chemical manufacturing plant, now a chemical superfund site, in Wilmington, MA, exposed local residents to high levels of NDMA through drinking water for several years. The exposure was only discovered through the unusual cluster of 22 children in a town with less than 22,000 people who got cancer. The drinking water was eventually tested, and NDMA was identified in a rare case where isolating a single type of prolonged chemical exposure could be linked to health outcomes.
MIT researchers followed up with studies on mice and determined that the child cancer rates were likely initiated in utero when cell division was highest. They also found an interesting pivot point for the effects of NDMA that were dependent on the levels of Alkyladenine DNA glycosylase (AAG) enzymes.
Too much cell division results in cancer when AAG is low versus cell death when AAG is high. Humans can vary by as much as 10-fold in their levels of AAG, requiring any study of NDMA effects to include signals of degenerative disease, something missing from the current study.
Who cares about a drug that is off the market?
First approved for U.S. markets in 1983, ranitidine (Zantac) was one of the first medications to reach $1 billion in sales and went on to generate $4 billion in yearly sales before being pulled from the market. Several drug companies have sold either the prescription, over the counter, branded or generic version of the drug before the FDA advised consumers to dispose of any ranitidine products. The recall damaged several company reputations, stock values and they have been fighting litigation and liability issues ever since.
The brand Zantac has been reformulated as Zantac 360º OTC and now contains famotidine, one of the FDA approved non NDMA forming H2RAs ranitidine was compared against in the current study.
It is unclear why the researchers chose to study ranitidine, a drug that is currently off the market and unlikely to return. In a statement about the limitations of the study the authors state, "The study period was relatively short and may underestimate the cancer risk associated with the longer-term use of ranitidine," and yet are still able to conclude that their findings "...do not support proactive cancer screening or surveillance among individuals previously exposed to ranitidine."
In the conflict of interest disclosures section, lead author Dr. Seng Chan You reported receiving personal fees from IQVIA, who curates the main databases used in the study. IQVIA also has an employee listed as an author of the paper who was involved in design, analysis, interpretation of data, and supervision of the study.
Receiving personal fees from IQVIA and having an IQVIA employee involved in the research is not uncommon in some pharmaceutical research circles. IQVIA is a leading global provider of advanced analytics, technology solutions and clinical research services to the life sciences industry and participation on some level might be expected, though it is worth noting that the employee involved in the current study has a strong "strategy" focus within IQVIA.
IQVIA also has a major brand marketing and promotion side to the company and their website contains typical examples of marketing strategy lingo that in most commercial contexts would be expected, but could be inappropriate in a research setting like "Leverage evidence to drive patient access at a price that maximizes value for health care stakeholders."
As with any research, transparency in potential conflicts of interest is critical. With the multifaceted ways that a company like IQVIA could be conflicted, a conflict of interest disclosure from them seems warranted on any study they are involved with.
More information: Seng Chan You et al, Ranitidine Use and Incident Cancer in a Multinational Cohort, JAMA Network Open (2023). DOI: 10.1001/jamanetworkopen.2023.33495
Bevin P. Engelward, Implications of an epidemiological study showing an association between in utero NDMA exposure and childhood cancer, Environmental and Molecular Mutagenesis (2021). DOI: 10.1002/em.22434
© 2023 Science X Network