This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Specialized T cells in the brain found to slow progression of Alzheimer's disease
As many as 5.8 million Americans are currently living with Alzheimer's disease, a neurodegenerative condition associated with progressive cognitive decline, including loss of memory capabilities. Protein aggregates, composed of beta-amyloid or other proteins, form in the brains of individuals with Alzheimer's.
These beta-amyloid plaques appear to be a significant contributor to the disease. St. Jude Children's Research Hospital scientists uncovered a subset of immune cells that appears to slow this beta-amyloid plaque accumulation and the key proteins involved in the process. The findings were published today in Nature Immunology.
"People typically think of the immune system as being involved in defense from bacterial or viral infection, though there is growing interest in the role of the immune system in neurodegenerative diseases," said co-first author Jordy Saravia, Ph.D., St. Jude Department of Immunology. "We uncovered an important immune cell communication axis that is protective in an Alzheimer's disease model."
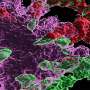
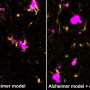
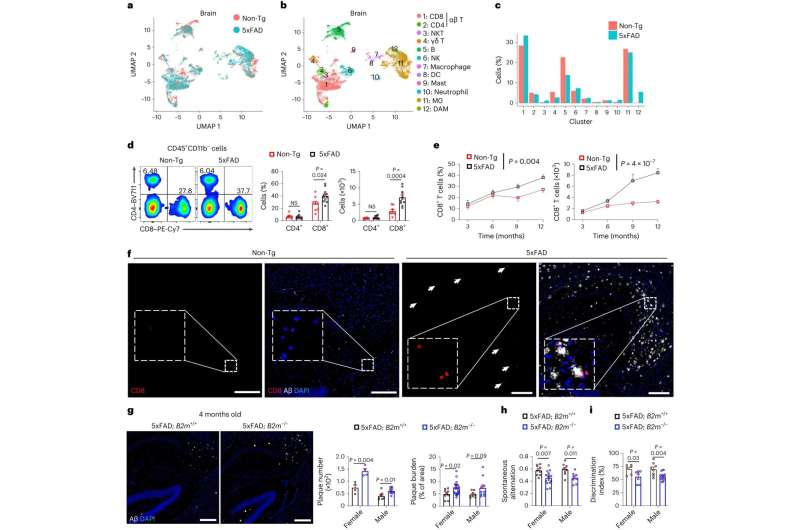
Microglia are immune cells in the brain responsible for clearing beta-amyloid plaques. As Alzheimer's disease progresses, microglia can lose their capacity to remove these plaques and instead produce inflammatory mediators that may accelerate beta-amyloid plaque progression. The St. Jude team found that accumulating another subtype of immune cells, called CD8+ T cells, is essential to slow this process by interacting with microglia. This interaction, in turn, was important to limit beta-amyloid burden and preserve memory capabilities in a mouse model of the disease.
"Our paper is the first to demonstrate that a subpopulation of CD8+ T cells can be protective in a mouse model of Alzheimer's disease," said co-first author Wei Su, Ph.D., St. Jude Department of Immunology. "Moving forward, we may be able to extend this work to find an effective intervention for neurodegenerative diseases."
Immune cells' opposing roles in Alzheimer's disease
Previous research has established complex roles for T cells and other immune system cells in Alzheimer's disease. In particular, research groups using other experimental systems have suggested that certain T cells with inflammatory functions worsen the disease. However, the St. Jude scientists showed that CD8+ T cells with suppressive features accumulate in the brains of both mouse models and patients with Alzheimer's disease, highlighting that T cells play a complex role in this disease.
"We showed that CD8+ T cells can play a protective role against Alzheimer's disease pathogenesis, although there is also evidence for a contributing role," said corresponding author Hongbo Chi, Ph.D., St. Jude Department of Immunology. "Our results demonstrate the need to better understand these complex neuro-immune interactions to improve outcomes for this neurodegenerative disease."
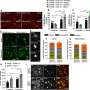
To understand how T cells were delaying symptom progression in their Alzheimer's disease model, the St. Jude group searched for the most abundant molecular interaction between CD8+ T cells and the microglia. They found a protein on the surface of CD8+ T cells, CXCR6, interacts with the protein CXCL16 expressed by microglia.
A molecular handshake slows Alzheimer's disease
The two surface proteins, CXCR6 and CXCL16, essentially performed a handshake between the two cells, communicating in both directions. Just like the firmness of a human handshake can convey information, so can the interaction of these two proteins on the outside of their respective cells.
"We found CD8+ T cells use CXCR6 to interact with CXCL16 from microglia," Chi said. "Moreover, CD8+ T-cell accumulation, localization and function in the brain are regulated by CXCR6."
The scientists determined how the handshake occurs and delays the onset of Alzheimer's disease-related pathologies. The CD8+ T cells first move next to the microglia, which are localized next to the beta-amyloid plaques. Then, the CD8+ T cells use the handshake to signal to the microglia to stop causing uncontrolled inflammation, which, in turn, slows plaque growth and symptoms in the mouse models.
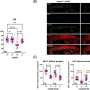
When the scientists deleted the gene for the CD8+ T cell's protein CXCR6, the mice developed worse Alzheimer's disease-related symptoms. This effect was partially because the CD8+ T cells without CXCR6 failed to accumulate in the brain near the microglia or plaque site. These cells also did not acquire the appropriate suppressive function. Thus, disrupting the CD8+ T cell's ability to perform the handshake prevented its protective effect against Alzheimer's disease symptoms.
"We have two major findings," Chi said. "One is the crucial role of CD8+ T cells in maintaining homeostasis of the brain, thereby providing a protective role in Alzheimer's disease." Homeostasis is the process of keeping a system in a relatively stable state. In this case, the CD8+ T cells attempt to limit the disruption caused by microglia dysfunction and Alzheimer's disease-related plaques.
"The other major finding is identifying the central importance of the T cell protein CXCR6 for CD8+ T-cell accumulation and function in the brain," Chi continued. "We really need to characterize these kinds of neuro-immune interactions better. Only by understanding this basic biology can we advance the field and find new treatments."
More information: Su, W. et al, CXCR6 orchestrates brain CD8+ T cell residency and limits mouse Alzheimer's disease pathology, Nature Immunology (2023). DOI: 10.1038/s41590-023-01604-z. www.nature.com/articles/s41590-023-01604-z