This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New technique helps CAR T therapies fight cancer cells while minimizing severe side effects
In recent years, cancer researchers have hailed the arrival of chimeric antigen receptor T cell (CAR T) therapy, which has delivered promising results, transforming the fight against various forms of cancer. The process involves modifying patients' T-cells to target cancer cells, resulting in remarkable success rates for previously intractable forms of cancer.
Six CAR T cell therapies have secured FDA approval, and several more are in the pipeline. However, these therapies come with severe and potentially lethal side effects, namely cytokine release syndrome (CRS) and neurotoxicity. These drawbacks manifest as a range of symptoms—from high fever and vomiting to multiple organ failure and patient death—posing significant challenges to broader clinical application.
Now, a research team led by Michael Mitchell, associate professor in the School of Engineering and Applied Science at the University of Pennsylvania, has found a solution that could help CAR T therapies reach their full potential while minimizing severe side effects. Their findings are published in the journal Nature Materials.
"Addressing CRS and neurotoxicity without compromising the therapeutic effectiveness of CAR T cells has been a complex challenge," says Mitchell.
He says that unwanted interactions between CAR T and immune cells called macrophages drive the overactivation of macrophages, which in turn result in the release of toxic cytokines that lead to CRS and neurotoxicity.
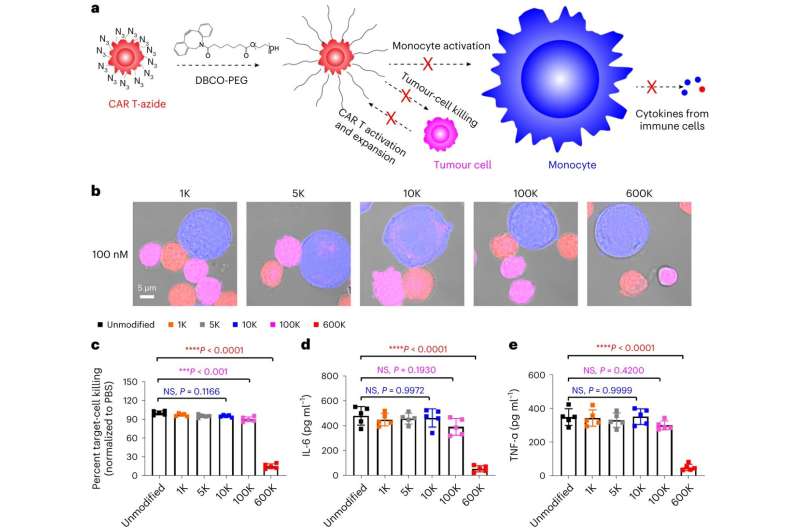
"Controlling CAR T–macrophage interactions in vivo is difficult," Mitchell says. "So, our study introduces a materials engineering-based strategy that involves incorporating a sugar molecule onto the surface of CAR T cells. These sugars are then used as a reactive handle to create a biomaterial coating around these cells directly in the body, which acts as a 'suit of armor,' preventing dangerous interactions with macrophages."
First author Ningqiang Gong, a postdoctoral researcher in the Mitchell Lab, elaborates on the technique, "We attached this sugar molecule to the CAR T cells using metabolic labeling. This modification enables the CAR T cells to attack cancer cells without any hindrance."
"When symptoms of CRS begin to manifest, we introduce another molecule—polyethylene glycol (PEG)—to create the suit of armor, which effectively blocks dangerous interactions between these engineered T cells, macrophages, and the tumor cells themselves," Gong says.
Over time, small tumor antigens can still reach what the researchers call "PEGylated CAR T cells," slowly activating and expanding them without triggering the severe side effects associated with rapid activation and expansion. As the CAR T cells slowly expand, the surface density of PEG becomes diluted, progressively restoring their ability to interact with other cells.
The team says that their approach offers more than just a safety net for patients; it also opens up a new "therapeutic window" for treatment.
This is made possible, Gong says, due to the size differences among tumor cells, CAR T cells, and macrophages. He says tumor cells and CAR T cells are typically smaller (ranging from 5 μm to 10 μm) compared to macrophages (>20 μm), and as the surface density of PEG on CAR T cells begins to dilute, interactions between CAR T cells and tumor cells are restored before interactions with macrophages.
This restoration says Mitchell allows CAR T cells to target and kill cancer cells without causing macrophage overactivation, thereby minimizing the risk of dangerous CRS symptoms and neurotoxic effects.
"By incorporating the PEG buffer, we've successfully managed to modulate the interactions between CAR T cells and macrophages. This enables a therapy that is both safer and effective," Mitchell says.
Beyond this, the team is also examining the potential for in situ PEGylation to be applied to other types of cellular immunotherapies and even broader applications. "The implications could be far-reaching," says Mitchell. "We're looking at a potentially universal approach to making cellular therapies safer for all patients."
More information: Ningqiang Gong et al, In situ PEGylation of CAR T cells alleviates cytokine release syndrome and neurotoxicity, Nature Materials (2023). DOI: 10.1038/s41563-023-01646-6