This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Targeting cancer's hidden master regulators
Cancer research has long focused on targeting the genetic mutations that drive tumors. Many of these genetic changes affect genes that allow mutated cells to replicate out of control. While developing drugs to target these mutations can benefit some patients, most tumors either lack targetable mutations or develop resistance to those therapies as a result of the accumulation of even more mutations.
It's an evolutionary arms race drug developers are bound to lose.
"The number of potential mutational patterns that may turn a cell cancerous is larger than the number of atoms in the universe and so it ends up being very difficult to develop mutation-targeting drugs that will avoid resistance," says Andrea Califano, Dr., professor of systems biology at Columbia University Vagelos College of Physicians and Surgeons.
However, despite the fact that virtually every tumor cell has a different mutational pattern, oncologists have long noticed that many tumors behave in quite similar ways and may respond to the same drugs.
"If tumors with different mutations behave in the same way, there must be some mechanisms that integrate the effect of the mutations to drive these tumor cells towards the same molecular state. This was the 'aha!' moment that led to the development of a new approach to identify effective therapies for cancer patients independent of their genetic mutations," says Califano, who for the past decade has been leading a team of more than 50 scientists in testing that strategy in patients with previously intractable or relapsed cancers.
Their study findings, which may shift the way cancer is treated in the future, were published in the journal Cancer Discovery.
First step: Find the mechanisms
To find these mechanisms, his lab combined experiments in cultured cells and animal models with computational analysis of lab results and patient data.
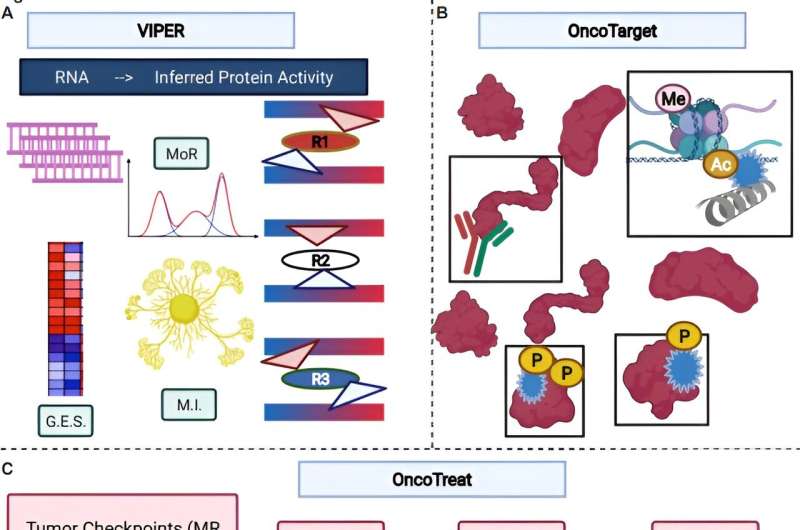
Over the course of more than a decade, those efforts uncovered a relatively small and highly modular set of "master regulators," representing regulatory proteins that are both necessary and sufficient to drive cancer growth, regardless of the specific pattern of mutations that activate them.
Thus, the virtually infinite number of mutations found across all tumors end up activating only about a hundred highly distinct master regulator modules, which can be targeted pharmacologically, an enormous reduction in complexity. Notably, master regulators themselves are rarely if ever mutated, which is why previous mutation-focused studies had failed to identify them.
Now, by examining the pattern of gene expression within the cells of a tumor, Califano's team can predict which master regulators are responsible for a specific tumor's growth. Looking at the effects of different chemotherapies, they can then predict which drugs would either directly target a key master regulator or override their global nefarious function by reversing the activity of an entire master regulator module, which can contain up to 50 proteins.
Since these modules act as a bottleneck that canalizes the effect of an incredibly large set of potential mutational patterns, "this strategy makes it very hard for the cancer cell to find a genetic recipe to bypass the effect of the drug," says Califano.
A different type of clinical study
As his team discovered master regulator modules for a large number of human cancers, Califano also began assembling protocols and collaborators for an unusual clinical study. Traditionally, clinical trials of cancer drugs recruit patients with a specific form of the disease and test one therapy to see if it works. To get statistically valid data, those trials must include a large number of patients, also known as the "N" of the study.
Instead, Califano envisioned an entirely different kind of study: enrolling patients with more than 20 different types of treatment-resistant cancer, without considering their mutations and predicting which drug, from more than 300 clinically relevant drugs, would be most likely to kill the tumor on an individual patient basis.
In this "N of 1" design, each patient essentially gets their own clinical trial. "This is a clinical trial in which you cannot assess the efficacy of one particular drug in one particular type of tumor because there wouldn't be enough patients. What you can do, however, is to use the trial to assess the efficacy of the methodology to predict drugs that are most likely to work," says Califano.
When recruitment started in 2013, the study was open to 18 different malignancies and eventually enrolled about 130 patients, growing to include virtually all tumor types. Because of the scope of the project and the breadth of expertise it required, he collaborated with researchers from multiple Columbia departments, as well as researchers from Emory University and Memorial Sloan Kettering Cancer Center.
For each patient, the team obtained tumor tissue and conducted detailed gene expression profiling on it. They then used their computational approach to determine which master regulators were driving the tumor's growth. To find potential treatments, they used one of two algorithms: OncoTarget, which identifies drugs expected to inhibit the master regulators of a tumor directly, or OncoTreat, which identifies drugs predicted to inactivate the entire master regulator module. Many of the drugs the algorithms identified had never been tested on those tumor types.
To check the predictions, the researchers transplanted a fragment from the patient's tumor in mice, thus ensuring that the transplanted tumors relied on the same master regulators as the ones in the patient.
About two-thirds of the drugs predicted to work by OncoTarget and 91% of those predicted to work by OncoTreat controlled the transplanted tumors, compared to 0% treated with other therapies.
"These tumors were derived from patients who had failed multiple lines of therapy," says Califano. "Not only did the drugs work in the animals, they appeared to act through exactly the same mechanisms predicted by our models, as shown by measuring the change in master regulators shortly after treatment."
Moving to the clinic
Though patients on the N of 1 study could not be treated using the predicted drugs, there have been several metastatic patients who have shown dramatic response to the predicted therapy in other studies. This includes patients with aggressive pediatric tumors and those in a recently completed clinical trial for metastatic breast cancer, in collaboration with colleagues at Emory, Mount Sinai, and St. Jude, where the drug identified by master regulator analysis was shown to be effective in 100% of the patients predicted to respond.
Conducting N of 1 studies on every cancer patient seeking treatment would be impossible, but the findings point to a more scalable strategy.
"What we found through these studies is that, in fact, patients really stratify into very large subgroups, called pharmacotypes, that have predicted sensitivity to exactly the same drugs, thus paving the road to more traditional clinical trials," says Califano.
He and his colleagues are now developing procedures to classify patients into pharmacotypes in the clinic. One such trial for metastatic rhabdoid and Wilms tumor has just opened up in collaboration with colleagues at Memorial Sloan Kettering and another one for neuroendocrine tumors was completed at Columbia.
"I think we're doing something that is very different from what people normally think in terms of personalized medicine, but it's personalized in a way that could actually really be brought to the clinic and have utility for a vast number of patients," says Califano.
Tackling cell variability
The tumors that didn't respond to the predicted treatments highlighted a different problem: Tumor cells can be highly variable. Analyzing the cell population as a whole reveals the master regulators acting in many of the tumor cells, but in some cases there are sub-populations that have switched to different master regulators. Those sub-populations can survive the initial treatment and repopulate the tumor. Califano says the team has now developed techniques to target sub-populations detected by single cell analyses.
Attacking the master regulatory program could also make other therapies much more effective. "When you target master regulators, you dramatically sensitize the cell to immunotherapy, because the programs that allow the cancer cell to become invisible to the immune system, for instance by recruiting immunosuppressive normal cells to the tumor site, are actually largely regulated by the same master regulators that control the state of the cancer cell," says Califano.
Califano's lab, in collaboration with Cory Abate-Shen, Ph.D., at VP&S, showed this in a study metastatic castration-resistant prostate cancer, where the drugs predicted to target the master regulators increased the effect of immunotherapy, an approach that has very limited success in this type of cancer.
Califano and many of the same collaborators have produced a series of other studies in the past year supporting the master regulator strategy, and the idea is gaining traction.
"We're working with some of the major pharmaceutical companies that are now shifting their focus towards developing drugs that target master regulators, as opposed to using master regulators as a biomarker for whether an existing drug will work," says Califano.
With clinical trials of master regulator-based therapy now enrolling or underway for several types of cancer, the strategy seems poised for wider use. "I think that this is reaching the point where progress is becoming much, much faster; hopefully, it will not take another 10 years to complete the next studies," says Califano.
More information: Prabhjot S. Mundi et al, A Transcriptome-Based Precision Oncology Platform for Patient–Therapy Alignment in a Diverse Set of Treatment-Resistant Malignancies, Cancer Discovery (2023). DOI: 10.1158/2159-8290.CD-22-1020