This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Developing the first drug for a deadly bone disease
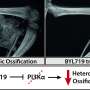
![(A) Distribution of non-transformed annualized new HO volume in individuals in MOVE and the NHS (Principal FASa). (B) Distribution of model-fitted non-transformed annualized new HO volume in individuals in MOVE and the NHS using a wLME model (Principal FASa). aRefers to patients in MOVE only. (A) The mean annualized new HO volume using non-transformed values was 60.3% lower in MOVE versus the NHS (MOVE: 9.4 × 103 mm3; NHS: 23.7 × 103 mm3). (B) wLME estimates are from a mixed model with the dependent variable being annualized new HO volume and independent variables including fixed effects of treatment, baseline total HO volume/baseline age, and a random patient effect, with observations weighted proportional to length of time between first and last WBCT. The wLME model estimated that the LSmean annualized new HO volume was 53.8% lower in MOVE versus the NHS (MOVE: 9.4 × 103 mm3; NHS: 20.3 × 103 mm3). wLME estimate of treatment effect: –10.9 × 103 mm3 (95% confidence interval [CI]: –21.2 × 103, –0.6 × 103; p = 0.039); wLME estimate of baseline total HO volume/baseline age effect: 0.3 mm3 (95% CI: –0.1, 0.7; p = 0.1440); wLME estimate of intercept effect: 16.2 × 103 mm3 (95% CI: 7.5 × 103, 24.9 × 103; p = 0.0003). FAS = Full Analysis Set; HO = heterotopic ossification; LS = least squares; NHS = natural history study; SEM = standard error of the mean; WBCT = whole-body computed tomography; wLME = weighted linear mixed-effects. Credit: Journal of Bone and Mineral Research (2022). DOI: 10.1002/jbmr.4762 UCSF research vital to first drug for deadly bone disease](https://scx1.b-cdn.net/csz/news/800a/2023/ucsf-research-vital-to.jpg)
The U.S. Food and Drug Administration (FDA) recently approved palovarotene (Sohonos) as the first treatment for fibrodysplasia ossifcans progressiva (FOP), a severely disabling condition that causes abnormal bone formation in place of soft and connective tissues. The approval was based on research conducted through a multicenter clinical trial, including patients being treated with the drug at UC San Francisco's Metabolic Bone Clinic.
FOP is a very rare genetic condition that leads to progressive loss of mobility as soft tissue painfully swells and bone forms where ligaments, fascia, tendons and joints should be—a process known as heterotopic ossification (HO).
The condition severely limits quality of life, with many patients being unable to move or care for themselves. Patients with FOP are often permanently bedridden or in a wheelchair by their 30s. Their life expectancy is shortened to an average of 56 years due to bone formation around the chest that leads to cardiorespiratory failure when the chest is unable to support normal breathing.
The FDA approval was based on results from the Phase III MOVE trial, a clinical trial for adult and pediatric patients. The trial included 107 patients from 16 international study sites in Argentina, Australia, Brazil, Canada, France, Italy, Japan, Spain, Sweden, the United Kingdom and the U.S., with the first patient enrolled in November 2017. The main part of the trial was conducted for 24 months and was followed with a two-year extension.
54% saw improvement
The study data was published in December 2022 in the Journal of Bone and Mineral Research showing that palovarotene significantly reduced the annual new HO volume compared with the current standard of care, which is mainly limited to symptom management and flare-up prevention. Treatment with palovarotene—an oral retinoid medication—reduced HO by 54% compared to patients in a FOP natural history study (which UCSF was also part of).
"The published Phase III MOVE study showed that palovarotene can decrease new heterotopic ossification, and that it can be tolerated by many patients with FOP," said co-first author Edward Hsiao, MD, Ph.D., a UCSF endocrinologist and professor of medicine specializing in metabolic bone diseases. "As a clinician caring for patients with FOP, I personally see the daily challenges and stresses that our patients and their families must contend with. Approval of this medication is a significant step towards improving their lives."
Patients in the study received an oral dose of palovarotene daily. At the onset of any flare-up—defined by the presence of at least one symptom including pain, swelling, redness, decreased range of motion, stiffness and warmth—patients received increased doses of palovarotene daily until the flare-up ended.
"Palovarotene is not for everyone with FOP," said Hsiao, noting that some young children treated with the drug developed severe complications including early growth plate closure and short stature. Palovarotene also had milder side effects for most study participants, including dryness of the skin and mucus membranes. But he added, "Since the accumulation of HO in FOP is progressive, irreversible and life altering, this medication is an important treatment option for our FOP community to consider."
Hsiao cares for patients with FOP at UCSF's Metabolic Bone Clinic, a unique resource within the endocrinology and metabolism division that focuses on the diagnosis and care of patients with inherited, rare or unusually complicated bone conditions. In addition to FOP, the clinic treats patients for congenital diseases and other skeletal dysplasias such as fibrous dysplasia/McCune-Albright syndrome, osteogenesis imperfecta and spondyloarthropathies.
Hsiao and his colleagues also treat endocrine and metabolic bone diseases that occur with conditions such as hyperparathyroidism, mitochondrial diseases and enzyme deficiencies. The team works closely with other divisions at UCSF, including medical genetics, orthopedics, nephrology, rheumatology, dentistry and craniofacial surgery to provide patients with the comprehensive care for their conditions. Hsiao also leads a research program focused on understanding and treating human bone diseases.
More information: Robert J. Pignolo et al, Reduction of New Heterotopic Ossification (HO) in the Open‐Label, Phase 3 MOVE Trial of Palovarotene for Fibrodysplasia Ossificans Progressiva (FOP), Journal of Bone and Mineral Research (2022). DOI: 10.1002/jbmr.4762