October 16, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
EarSkin and EarCartilage—combining bioengineered human skin with bioprinted cartilage for ear reconstruction
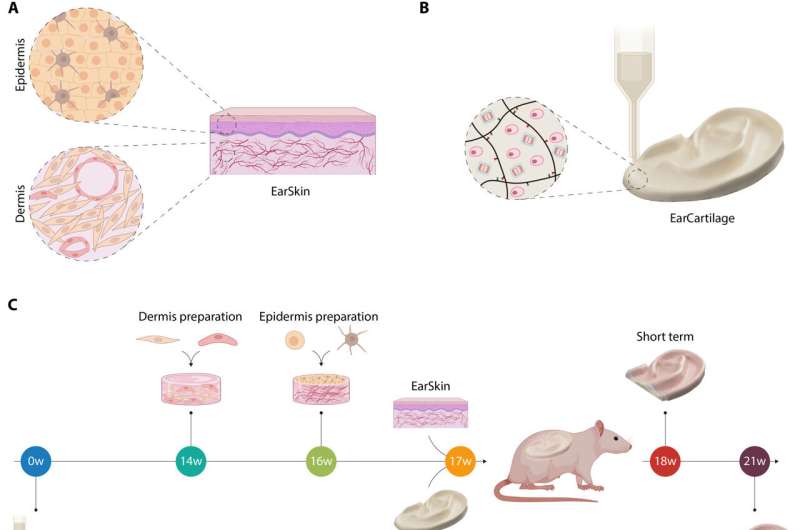
Microtia is a congenital disorder that can occur as a malformation of the external ear in children. In a new study published in Science Advances, Dominika Zielinska and a research team in tissue biological research, tissue engineering, polymer technologies and biofabrication at the University of Children's Hospital Zurich, ETH Zurich, Switzerland and the U.S., developed a tissue-engineered treatment approach by using bioprinted autologous auricular cartilage construct as ear cartilage, combined with a bioengineered human pigmented and prevascularized ear skin substrate already tested in immunocompromised rats.
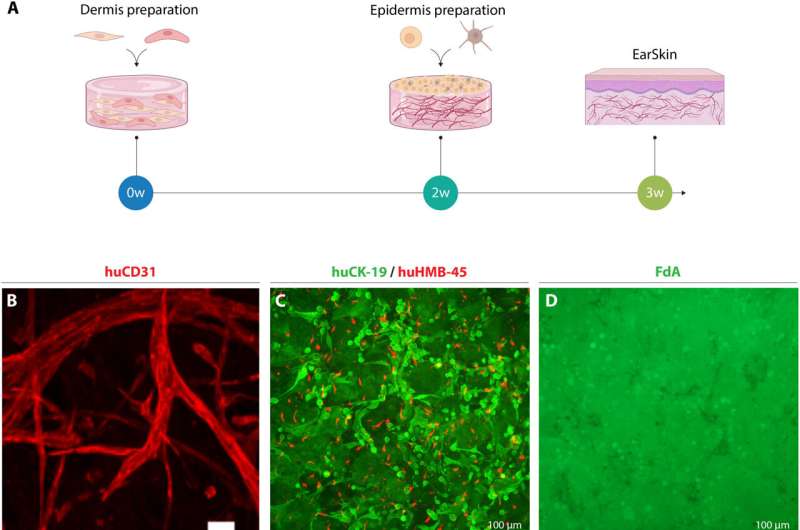
The team confirmed the capacity of the human engineered blood capillaries of EarSkin to connect to the recipient's vasculature within a week for rapid blood perfusion and epidermal maturation. The bioengineered EarSkin demonstrated a stratified epidermis with mature keratinocytes and melanocytes, where the latter resided within the basal layer of the epidermis to efficiently restore the skin color.
Additional in vivo tests showed favorable mechanical stability of the ear cartilage accompanied with improved extracellular matrix deposition. The combined trademarks of Earskin and EarCartilage represented a new treatment approach to microtia, to overcome limits and improve the aesthetic outcomes of microtia reconstruction.
Characterizing microtia for EarSkin and EarCartilage tissue engineered grafts
During congenital conditions, microtia can be characterized by the abnormal development of the external ear at the fetal stage, where it ranges from minor deformities to a complete lack of the auricle. Microtia affects a broad population of children worldwide, affecting their psychosocial well-being, while treatment strategies only become available by the age of 10. The gold standard to treat microtia is autologous costal cartilage reconstruction, where the costal cartilage can be harvested from the ribcage to carve out an auricular graft framework.
This construct is then implanted under the skin of the skull to elevate the auricle in a second stage in a highly complex plastic surgery procedure. The intervention required harvesting a sufficient amount of costal cartilage and is therefore usually delayed until children are 10 years of age.
While scaffold implantation with porous high-density polyethylene can circumvent costal cartilage harvesting, the implants suffered from higher complication rates at the site. In this work, the team combined human pigmented and prevascularized skin substitute (Earskin) with bioprinted cartilage (EarCartilage) as a first attempt to use two tissue-engineered grafts to treat microtia patients without complications.
Evaluating the EarSkin in the lab and developing bioprinted human cartilage in vitro
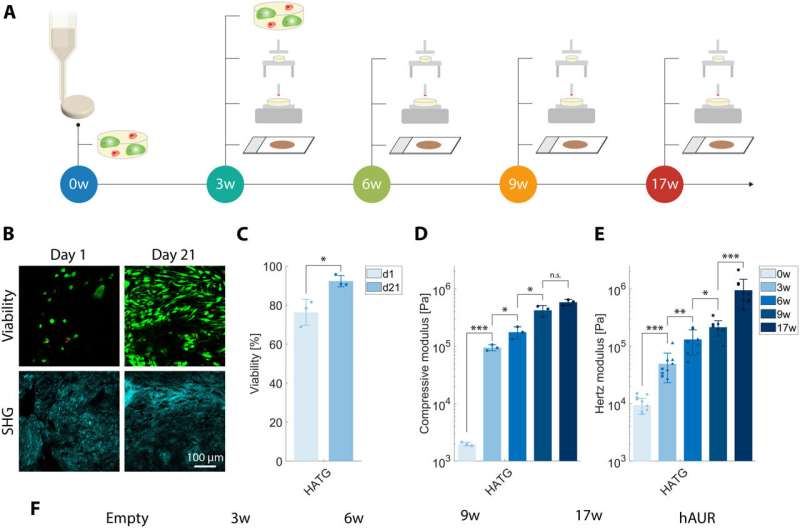
To develop the bioengineered EarSkin, the team incorporated a dermo-epidermal skin graft of human origin based on a collagen type I hydrogel with a prevascularized dermal layer and a pigmented dermis on top. The team co-cultured human dermal microvascular endothelial cells with human fibroblasts in the dermis by forming branching capillaries arranged into a vascular plexus within two weeks of culture in vitro. The capillary number and capillary length increased significantly in time.
The tissue maintained a complex extracellular matrix composition where the cartilage withstood significant loads. The team tested the maturation of cartilage constructs after bioprinting the cellular disks and studying their mechanical properties to show the extracellular matrix of tissue engineered constructs owing to the mature elastic network of the human auricular cartilage.
Combining the EarSkin with cartilage disks in vivo
The scientists developed an in vivo rat model to mimic the combined engineered skin and cartilage to treat microtia. They established the model by performing a first experiment by implanting disks under the panniculus carnosus, used clinically in ear reconstitution and transplanted with prevascularized dermo-epidermal skin grafts on top. The team regulated the wound healing process after implantation weekly and documented the process photographically to detail macroscopic skin maturation.
Then using histological studies, the researchers assessed the EarSkin panniculus carnosus cartilage disks, and noted the presence of human capillaries in the dermal layer of EarSkin with encompassed fibroblasts while the cartilage disks remained avascular. Then by using histology and immunohistology studies they confirmed tissue maturation of the cartilaginous disks. The outcomes emphasized the significance of the maturation process during the culture timeframe.
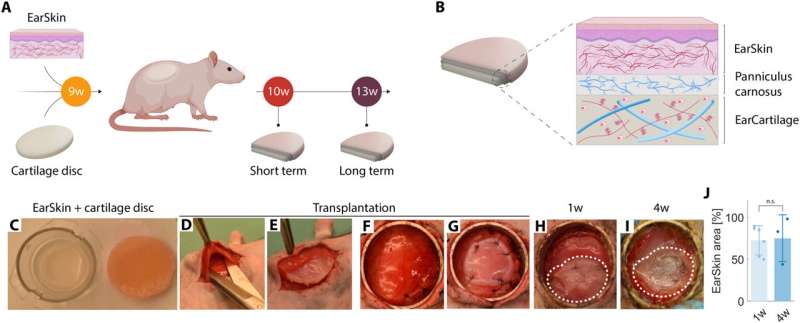
Combining EarSkin with EarCartilage in an animal model
Zielinska and colleagues used the EarCartilage as a close-to-physiological 3D topography for the panniculus carnosus and EarSkin. They combined the two biologically engineered structures and assessed their functionality. After transplantation, they continued the wound healing process and studied EarSkin maturation.
Before implanting the construct, the EarCartilage displayed a flexible and elastic behavior with a compressive modulus. By one to four weeks in vivo, the team preserved the morphology of the EarCartilage alongside its structure, where the auricular constructs remained significantly softer. Using histological and immunohistochemistry outcomes, they confirmed the deposition of collagen and elastin variants.
Outlook
In this way, Dominika Zielinska and team successfully combined two tissue-engineered grafts created in the lab in the form of a tissue engineered human skin graft (EarSkin) and bioprinted human cartilage graft (EarCartilage). The approach presented an innovative and promising treatment strategy for patients born with microtia, and the work can overcome costal cartilage harvesting to facilitate the intervention at an earlier age as well as provide a personalized aesthetic surgical option for microtia based on patient-derived cells.
During in vivo experiments in an animal model, the team used the panniculus carnosus; a highly vascularized muscle layer as an equivalent to the human temporal fascia flap.
The scientists observed a remodeling process of the transplanted human vasculature in vivo with human engineered capillary networks, as early as four weeks after transplantation. The EarSkin–EarCartilage constructs were stable in vivo with shape retention throughout implantation, demonstrating the successful combination of two tissue-engineered constructs of 3D cartilage and skin as a personalized treatment strategy for childhood microtia.
More information: Dominika Zielinska et al, Combining bioengineered human skin with bioprinted cartilage for ear reconstruction, Science Advances (2023). DOI: 10.1126/sciadv.adh1890
Benjamin P. Cohen et al, Tissue engineering the human auricle by auricular chondrocyte-mesenchymal stem cell co-implantation, PLOS ONE (2018). DOI: 10.1371/journal.pone.0202356
© 2023 Science X Network