This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
A promising target to fight inflammatory bowel diseases
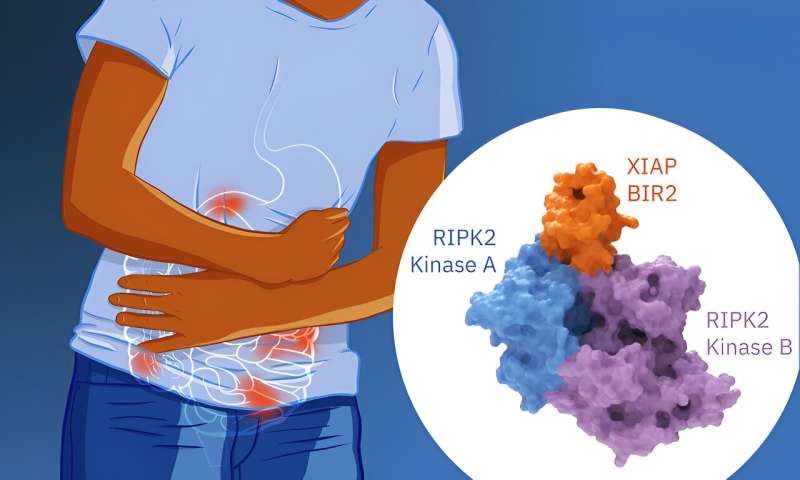
Inflammatory bowel diseases (IBDs), such as Crohn's disease or ulcerative colitis, arise when there is a dysregulation of the cell signaling pathways controlling the maintenance of homeostasis in the gut, leading to a chronic inflammatory response.
Researchers from the Cusack group have provided insights into the interactions of two molecules—XIAP and RIPK2—involved in these signaling pathways.
Signaling pathways in the gut: A delicate balance
Inflammation is a useful immune response to the constant exposure of cells to stressors such as pathogens. However, too much signaling leads to an undesirable amount of inflammation, which, in consequence, affects the organism's normal functioning.
The gut inflammatory response involves a complex machinery that gives rise to a chain reaction involving several molecules. NOD1 and NOD2 are receptors in charge of identifying the bacterial fragments that we are exposed to. When they recognize these fragments, they start the "fight back" response by activating the RIPK2 kinase.
This kinase then "recruits" another molecule, called XIAP, which reacts by attaching a specific chain of amino acids (the ubiquitin chain) to RIPK2. This reaction is necessary for the recruitment of more proteins to trigger the inflammatory response.
"This signaling pathway keeps under control the bacteria in your gut. It's very finely balanced because you don't want to kill all the bacteria but you don't want them to grow too much either," said Stephen Cusack, co-corresponding author of the publication. "Inflammatory bowel diseases tend to arise when there is a dysregulation of this NOD2 signaling and of the whole downstream chain reaction."
One way to treat these diseases is, therefore, to target proteins in the signaling pathway to keep things under control. Erika Pellegrini, former EMBL staff researcher in the Cusack group and co-corresponding author, has worked on such targets since 2013. "When I started my project on RIPK2, it was clear that this kinase was a good target for IBDs because it is only involved in the NOD1/NOD2 signaling pathways," explained Pellegrini. "A drug targeting this kinase would block this signaling pathway without affecting other cellular processes."
Pellegrini's research focused on how RIPK2's upstream and downstream interactions affect inflammatory signaling pathways. In a paper published in Life Science Alliance, Pellegrini and colleagues share insights on the interactions between RIPK2 and XIAP—a challenging target because of the dynamic and unusually small nature of this protein complex.
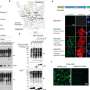
Thanks to cryo-electron microscopy, Pellegrini and Mathilde Lethier, first author of the publication, managed to obtain the 3D structure of XIAP/RIPK2 complex and found out how XIAP uses a specific domain (BIR2) to interact with RIPK2. Two molecules of the RIPK2 domain are arranged in an antiparallel dimer and the structure reveals that XIAP binds to both RIPK2 molecules.
"The structure of RIPK2 kinase bound to the XIAP BIR2 domain reveals the essential role of the RIPK2 dimer in NOD2 signaling and can explain the mechanism of already known anti-inflammatory drugs inhibiting the interactions of these two molecules," explained Pellegrini.
Interest from the pharmaceutical industry
These results—when combined with previous structural biology studies on RIPK2 by Pellegrini in 2017 and 2018 —offer useful information to academics and pharmaceutical companies working on developing therapeutics to treat inflammatory bowel diseases.
Pellegrini and Cusack are currently collaborating with Oncodesign Precision Medicine (OPM), a clinical-stage French biotechnology company headquartered in Dijon. OPM is actively engaged in the development of a groundbreaking category of RIPK2 inhibitors, intended as a potential therapeutic approach to address unmet needs, particularly in the case of IBDs patients. Pellegrini and Cusack are providing structural data that sheds light on the interactions between OPM's RIPK2 inhibitors and the kinase domain of RIPK2.
"There are still plenty of unknown questions about the structure of interacting components of these signaling pathways, and answering these would help us understand the many more steps in the chain reaction," added Cusack. "However, I think these studies on RIPK2 are very promising for drug development targeting inflammatory bowel diseases, and even cancer, as RIPK2 is also a cancer target."
More information: Mathilde Lethier et al, Structure shows that the BIR2 domain of E3 ligase XIAP binds across the RIPK2 kinase dimer interface, Life Science Alliance (2023). DOI: 10.26508/lsa.202201784