This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study: How nutrients are used reprograms immune cells with implications for infection and cancer
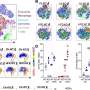
![Inhibiting choline metabolism drives pronounced changes in gene transcription in naïve and IL-4-polarized macrophages. A) Schematic of bulk RNA-seq sample preparation. Heatmap of z-scores for select genes of interest. n = 3. B) Volcano plots of comparisons between Veh and HC3 or RSM in M[0] or M[IL-4]. Red dots represent genes with false discovery rate (FDR) < 0.05 and log2 fold change (FC) < -1 or > 1. Select genes of interest are annotated. C-D) The top significantly up- or down-regulated genes shared in all comparisons in B) were analyzed by Enrichr-KG to identify common pathways. Schematics were created using BioRender. Credit: PLOS Pathogens (2023). DOI: 10.1371/journal.ppat.1011658 Study: How nutrients are used reprograms immune cells with implications for infection and cancer](https://scx1.b-cdn.net/csz/news/800a/2023/study-how-nutrients-ar.jpg)
A new study spearheaded by the University of Ottawa's Faculty of Medicine lab has unveiled a previously unrecognized role for an essential nutrient in shaping the cellular landscape for one of the body's first lines of defense against infection, immune cells called macrophages.
The findings identifying a role for the nutrient choline under normal conditions and in response to an intestinal worm infection in mice could potentially have significant implications for other models of human infection, perhaps even cancer immunity.
In the study published in PLOS Pathogens, Dr. Morgan Fullerton explains that by blocking a specific part of metabolism the research team saw an unexpected defect emerge in macrophages—sentinel cells that are one of the immune system's star players. Macrophages are known for their Pac Man-like capacity to gobble up microbes and cellular debris amid a coordinated immune response, ultimately repairing injured tissue.
"When we take away the ability of these cells to use a nutrient called choline, their regular response is blunted. We also found that blocking the use of choline with a drug, made the number and type of immune cells in mice were very different—with and without a parasite infection," says Dr. Fullerton, one of the study's senior authors and co-director of uOttawa's Centre for Infection, Immunity and Inflammation.
First author Dr. Peyman Ghorbani, a Postdoctoral Fellow in Dr. Fullerton's uOttawa lab, says this finding may be related to changes in macrophages' ability to generate energy through its mitochondria, shape-shifting organelles that act as the powerpacks inside our cells.
While exploring the links between metabolism and immunity, one goal of Dr. Fullerton's lab is to expand global knowledge regarding choline metabolism in immune cells. Choline is an essential nutrient found in a wide variety of foods including eggs, wheat germ and meats. And in the body, it's converted into a neurotransmitter called acetylcholine and is also metabolized in the liver.
For this specific work, mice infected with an intestinal worm were treated with an inhibitor of choline metabolism in vivo. The team found that there was a "tremendous reprogramming of the immune profile in the mice," according to Dr. Fullerton.
He says these findings suggest that immune and non-immune cells can be reprogrammed, both in their function and in their numbers, by changing how nutrients are used. This knowledge could perhaps eventually prove to have ramifications for battling cancer.
"Certain cancer therapies are geared toward turning on the immune system so it can fight cancer cells, like an infection," Dr. Fullerton says. "It might be that changing levels of choline with certain drugs, could influence these conditions. This would need to be tested in cells and in mice."
Future strategies could involve genetic and tissue-specific models to further demonstrate the importance of choline uptake and subsequent metabolism.
This was a deeply collaborative study. Dr. Fullerton's lab started working on macrophage cells and found that blocking choline led to less production of a specific cytokine—tiny proteins important in cell signaling—known as RELM alpha. To better understand the physiological implications of what they were observing in cells, they contacted Dr. Meera Nair, a scientist colleague at University of California, Riverside, who is an expert in mouse parasite models that lead to increases in RELM alpha.
Dr. Nair's lab ran the mouse infection studies, led by co-first author Dr. Sang Yong Kim. At the uOttawa Faculty of Medicine, the team benefited from the expertise of the labs of Dr. Steffany Bennett for lipidomic profiling, Dr. Julie St-Pierre for metabolism, Dr. Baptiste Lacoste for transmission electron microscopy, and Dr. Alexandre Blais for bioinformatics. The Faculty's cutting edge core facilities were also indispensable.
"We are very thankful for everyone's willingness to collaborate and for their expertise," says Dr. Fullerton.
More information: Peyman Ghorbani et al, Choline metabolism underpins macrophage IL-4 polarization and RELMα up-regulation in helminth infection, PLOS Pathogens (2023). DOI: 10.1371/journal.ppat.1011658